Cloning and functional characterization of the styE gene, involved in styrene transport in Pseudomonas putida CA-3
- PMID: 16461680
- PMCID: PMC1392900
- DOI: 10.1128/AEM.72.2.1302-1309.2006
Cloning and functional characterization of the styE gene, involved in styrene transport in Pseudomonas putida CA-3
Abstract
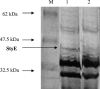
A 1.5-kb region immediately downstream of the styABCD operon involved in styrene degradation in Pseudomonas putida CA-3 has been cloned. Sequence analysis revealed a 1,296-bp open reading frame, designated styE, and BLAST P database comparisons of the deduced StyE amino acid sequence revealed 33 to 98% identity with several membrane-associated ATPase-dependent kinase proteins involved in the active transport of aromatic hydrocarbons across bacterial membranes and also with FadL, an outer membrane protein necessary for the uptake of long-chain fatty acids in Escherichia coli. Transcription of styE is styrene dependent, and the gene is cotranscribed with the styABCD structural genes. StyE appears to be membrane associated, with a corresponding 45.9-kDa band being identified following sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis of membrane preparations from styrene-grown cells. P. putida CA-3 cells in which the styE gene had been interrupted were no longer capable of growth on styrene. In contrast, overexpression of styE in P. putida CA-3 resulted in a 4.2-fold increase in styrene monooxygenase activity compared with wild-type cells grown on styrene, with a concomitant 8-fold increase in styA mRNA transcript levels. Experiments with the classic, ATPase inhibitor vanadate revealed that growth of wild-type cells on styrene was inhibited at a concentration of 1 mM, while 1.75 mM was required to achieve a similar effect in the StyE overexpression strain. Growth of either strain on citrate was not inhibited in the presence of up to 7 mM vanadate. These findings suggest a role for StyE in the active transport of styrene in Pseudomonas putida CA-3 and identify styrene transport as a potentially limiting factor with respect to mRNA transcript levels and associated enzymatic activity of the styrene degradative pathway.
Figures



Similar articles
-
Genetic characterization of accumulation of polyhydroxyalkanoate from styrene in Pseudomonas putida CA-3.Appl Environ Microbiol. 2005 Aug;71(8):4380-7. doi: 10.1128/AEM.71.8.4380-4387.2005. Appl Environ Microbiol. 2005. PMID: 16085828 Free PMC article.
-
Genetic characterization of the styrene lower catabolic pathway of Pseudomonas sp. strain Y2.Gene. 2003 Nov 13;319:71-83. doi: 10.1016/s0378-1119(03)00794-7. Gene. 2003. PMID: 14597173
-
Aromatic and aliphatic hydrocarbon consumption and transformation by the styrene degrading strain Pseudomonas putida CA-3.FEMS Microbiol Lett. 2005 Aug 15;249(2):267-73. doi: 10.1016/j.femsle.2005.06.016. FEMS Microbiol Lett. 2005. PMID: 16002236
-
Regulation of phenylacetic acid uptake is σ54 dependent in Pseudomonas putida CA-3.BMC Microbiol. 2011 Oct 13;11:229. doi: 10.1186/1471-2180-11-229. BMC Microbiol. 2011. PMID: 21995721 Free PMC article.
-
Biochemistry, genetics and physiology of microbial styrene degradation.FEMS Microbiol Rev. 2002 Nov;26(4):403-17. doi: 10.1111/j.1574-6976.2002.tb00622.x. FEMS Microbiol Rev. 2002. PMID: 12413667 Review.
Cited by
-
MhbT is a specific transporter for 3-hydroxybenzoate uptake by Gram-negative bacteria.Appl Environ Microbiol. 2012 Sep;78(17):6113-20. doi: 10.1128/AEM.01511-12. Epub 2012 Jun 22. Appl Environ Microbiol. 2012. PMID: 22729544 Free PMC article.
-
Salt Adaptation and Evolutionary Implication of a Nah-related PAHs Dioxygenase cloned from a Halophilic Phenanthrene Degrading Consortium.Sci Rep. 2017 Oct 2;7(1):12525. doi: 10.1038/s41598-017-12979-z. Sci Rep. 2017. PMID: 28970580 Free PMC article.
-
A beta-barrel outer membrane protein facilitates cellular uptake of polychlorophenols in Cupriavidus necator.Biodegradation. 2010 Jun;21(3):431-9. doi: 10.1007/s10532-009-9313-8. Epub 2009 Nov 24. Biodegradation. 2010. PMID: 19937267 Free PMC article.
-
Functional characterization of a StyS sensor kinase reveals distinct domains associated with intracellular and extracellular sensing of styrene in P. putida CA-3.Bioengineered. 2014 Mar-Apr;5(2):114-22. doi: 10.4161/bioe.28354. Epub 2014 Feb 26. Bioengineered. 2014. PMID: 24637704 Free PMC article.
-
On the Enigma of Glutathione-Dependent Styrene Degradation in Gordonia rubripertincta CWB2.Appl Environ Microbiol. 2018 Apr 16;84(9):e00154-18. doi: 10.1128/AEM.00154-18. Print 2018 May 1. Appl Environ Microbiol. 2018. PMID: 29475871 Free PMC article.
References
-
- Ausubel, F. M., J. G. Seidman, J. A. Smith, and K. Struhl. 1987. Current protocols in molecular biology. Greene Publishing Associates and Wiley Interscience, New York, N.Y.
-
- Bendtsen, J. D., H. Nielsen, G. von Heijne, and S. Brunak. 2004. Improved prediction of signal peptides: SignalP 3. J. Mol. Biol. 340:783-795. - PubMed
-
- Bradford, M. M. 1976. A rapid and sensitive method for the quantification of microgram quantities of protein utilising the principle of protein-dye binding. Anal. Biochem. 72:248-254. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

