Enhanced benzaldehyde tolerance in Zymomonas mobilis biofilms and the potential of biofilm applications in fine-chemical production
- PMID: 16461720
- PMCID: PMC1392954
- DOI: 10.1128/AEM.72.2.1639-1644.2006
Enhanced benzaldehyde tolerance in Zymomonas mobilis biofilms and the potential of biofilm applications in fine-chemical production
Erratum in
- Appl Environ Microbiol. 2006 Aug;72(8):5678
Abstract
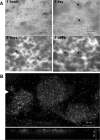
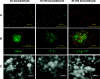
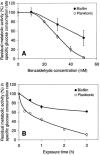
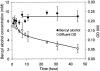
Biotransformation plays an increasingly important role in the industrial production of fine chemicals due to its high product specificity and low energy requirement. One challenge in biotransformation is the toxicity of substrates and/or products to biocatalytic microorganisms and enzymes. Biofilms are known for their enhanced tolerance of hostile environments compared to planktonic free-living cells. Zymomonas mobilis was used in this study as a model organism to examine the potential of surface-associated biofilms for biotransformation of chemicals into value-added products. Z. mobilis formed a biofilm with a complex three-dimensional architecture comprised of microcolonies with an average thickness of 20 microm, interspersed with water channels. Microscopic analysis and metabolic activity studies revealed that Z. mobilis biofilm cells were more tolerant to the toxic substrate benzaldehyde than planktonic cells were. When exposed to 50 mM benzaldehyde for 1 h, biofilm cells exhibited an average of 45% residual metabolic activity, while planktonic cells were completely inactivated. Three hours of exposure to 30 mM benzaldehyde resulted in sixfold-higher residual metabolic activity in biofilm cells than in planktonic cells. Cells inactivated by benzaldehyde were evenly distributed throughout the biofilm, indicating that the resistance mechanism was different from mass transfer limitation. We also found that enhanced tolerance to benzaldehyde was not due to the conversion of benzaldehyde into less toxic compounds. In the presence of glucose, Z. mobilis biofilms in continuous cultures transformed 10 mM benzaldehyde into benzyl alcohol at a steady rate of 8.11 g (g dry weight)(-1) day(-1) with a 90% molar yield over a 45-h production period.
Figures
References
-
- Allison, D. G., and I. W. Sutherland. 1984. A staining technique for attached bacteria and its correlation to extracellular carbohydrate production. J. Microbiol. Methods 2:93-99.
-
- Barrow, K. D., J. G. Collins, P. L. Rogers, and G. M. Smith. 1984. The structure of a novel polysaccharide isolated from Zymomonas mobilis determined by nuclear magnetic resonance spectroscopy. Eur. J. Biochem. 145:173-179. - PubMed
-
- Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. - PubMed
-
- Bringer-Meyer, S., and H. Sahm. 1988. Acetoin and phenylacetylcarbinol formation by the pyruvate decarboxylases of Zymomonas mobilis and Saccharomyces carlsbergensis. Biocatalysis 1:321-331.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

