Injectable, sustained-release naltrexone for the treatment of opioid dependence: a randomized, placebo-controlled trial
- PMID: 16461865
- PMCID: PMC4200530
- DOI: 10.1001/archpsyc.63.2.210
Injectable, sustained-release naltrexone for the treatment of opioid dependence: a randomized, placebo-controlled trial
Abstract
Context: Oral naltrexone can completely antagonize the effects produced by opioid agonists. However, poor compliance with naltrexone has been a major obstacle to the effective treatment of opioid dependence.
Objective: To evaluate the safety and efficacy of a sustained-release depot formulation of naltrexone in treating opioid dependence.
Design and setting: Randomized, double-blind, placebo-controlled, 8-week trial conducted at 2 medical centers.
Participants: Sixty heroin-dependent adults.
Interventions: Participants were stratified by sex and years of heroin use (> or = 5 vs < 5) and then were randomized to receive placebo or 192 or 384 mg of depot naltrexone. Doses were administered at the beginning of weeks 1 and 5. All participants received twice-weekly relapse prevention therapy, provided observed urine samples, and completed other assessments at each visit.
Main outcome measures: Retention in treatment and percentage of opioid-negative urine samples.
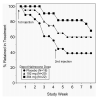
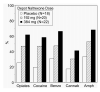
Results: Retention in treatment was dose related, with 39%, 60%, and 68% of patients in the placebo, 192 mg of naltrexone, and 384 mg of naltrexone groups, respectively, remaining in treatment at the end of 2 months. Time to dropout had a significant main effect of dose, with mean time to dropout of 27, 36, and 48 days for the placebo, 192 mg of naltrexone, and 384 mg of naltrexone groups, respectively. The percentage of urine samples negative for opioids, methadone, cocaine, benzodiazepines, and amphetamine varied significantly as a function of dose. When the data were recalculated without the assumption that missing urine samples were positive, a main effect of group was not found for any drugs tested except cocaine, where the percentage of cocaine-negative urine samples was lower in the placebo group. Adverse events were minimal and generally mild. This formulation of naltrexone was well tolerated and produced a robust, dose-related increase in treatment retention.
Conclusion: These data provide new evidence of the feasibility, efficacy, and tolerability of long-lasting antagonist treatments for opioid dependence.
Figures
Comment in
-
Lack of information in naltrexone study.Arch Gen Psychiatry. 2007 Jul;64(7):865; author reply 865. doi: 10.1001/archpsyc.64.7.865-a. Arch Gen Psychiatry. 2007. PMID: 17606820 No abstract available.
References
-
- Substance Abuse and Mental Health Services Administration (SAMHSA) Office of Applied Studies, Emergency department trends from the drug abuse warning network, Preliminary Estimates January-June 2001 with Revised estimates 1994 to 2000. Rockville, MD: 2002a. (DAWN Series D-20). DHHS Publication No. (SMA) 02-3634.
-
- Substance Abuse and Mental Health Services Administration. (SAMSHA) Summary of National Findings Office of Applied Studies. Volume I. Rockville, MD: 2002c. Results from the 2001 National Household Survey on Drug Abuse. (NHSDA Series H-17). DHHS Publication No. SMA 02-3758.
-
- Substance Abuse and Mental Health Services Administration (SAMHSA) Technical Appendices and Selected Data Tables. Office of Applied Studies. Volume II. Rockville, MD: 2002d. Office of Applied Studies, Results From the 2001 National Household Survey on Drug Abuse. (NHSDA Series H-18). DHHS Publication No. SMA 02-3759.
-
- Zacny J, Bigelow G, Compton P, Foley K, Iguchi M, Sannerud C. College on Problems of Drug Dependence taskforce on prescription opioid non-medical use and abuse: Position statement. Drug and Alcohol Dependence. 2003;69:215–232. - PubMed
-
- Bickel WK, Stitzer ML, Bigelow GE, Liebson IA, Jasinski DR, Johnson RE. Buprenorphine: Dose-related blockade of opioid challenge effects in opioid dependent humans. J Pharmacol Exp Ther. 1988;247:47–53. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical