Lateral mobility of proteins in liquid membranes revisited
- PMID: 16461891
- PMCID: PMC1413751
- DOI: 10.1073/pnas.0511026103
Lateral mobility of proteins in liquid membranes revisited
Abstract
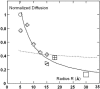
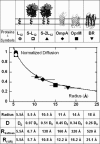
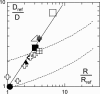
The biological function of transmembrane proteins is closely related to their insertion, which has most often been studied through their lateral mobility. For >30 years, it has been thought that hardly any information on the size of the diffusing object can be extracted from such experiments. Indeed, the hydrodynamic model developed by Saffman and Delbrück predicts a weak, logarithmic dependence of the diffusion coefficient D with the radius R of the protein. Despite widespread use, its validity has never been thoroughly investigated. To check this model, we measured the diffusion coefficients of various peptides and transmembrane proteins, incorporated into giant unilamellar vesicles of 1-stearoyl-2-oleoyl-sn-glycero-3-phosphocholine (SOPC) or in model bilayers of tunable thickness. We show in this work that, for several integral proteins spanning a large range of sizes, the diffusion coefficient is strongly linked to the protein dimensions. A heuristic model results in a Stokes-like expression for D, (D proportional, variant 1/R), which fits literature data as well as ours. Diffusion measurement is then a fast and fruitful method; it allows determining the oligomerization degree of proteins or studying lipid-protein and protein-protein interactions within bilayers.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures

References
-
- Reits E. A. J., Neefjes J. J. Nat. Cell Biol. 2001;3:145–147. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

