Adipocyte enhancer-binding protein 1 is a potential novel atherogenic factor involved in macrophage cholesterol homeostasis and inflammation
- PMID: 16461908
- PMCID: PMC1413702
- DOI: 10.1073/pnas.0508139103
Adipocyte enhancer-binding protein 1 is a potential novel atherogenic factor involved in macrophage cholesterol homeostasis and inflammation
Abstract
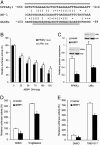
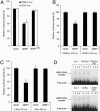
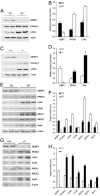
Peroxisome proliferator-activated receptor gamma1 (PPARgamma1) and liver X receptor alpha (LXRalpha) play pivotal roles in macrophage cholesterol homeostasis and inflammation, key biological processes in atherogenesis. Herein we identify adipocyte enhancer-binding protein 1 (AEBP1) as a transcriptional repressor that impedes macrophage cholesterol efflux, promoting foam cell formation, via PPARgamma1 and LXRalpha down-regulation. Contrary to AEBP1 deficiency, AEBP1 overexpression in macrophages is accompanied by decreased expression of PPARgamma1, LXRalpha, and their target genes ATP-binding cassette A1, ATP-binding cassette G1, apolipoprotein E, and CD36, with concomitant elevation in IL-6, TNF-alpha, monocyte chemoattractant protein 1, and inducible NO synthase levels. AEBP1, but not the C-terminally truncated DNA-binding domain mutant (AEBP1DeltaSty), represses PPARgamma1 and LXRalpha in vitro. Expectedly, AEBP1-overexpressing transgenic (AEBP1TG) macrophages accumulate considerable amounts of lipids compared with AEBP1 nontransgenic macrophages, making them precursors for foam cells. Indeed, AEBP1-overexpressing transgenic macrophages exhibit diminished cholesterol efflux compared with AEBP1 nontransgenic macrophages, whereas AEBP1-knockout (AEBP1-/-) macrophages exhibit enhanced cholesterol efflux compared with wild-type (AEBP1+/+) macrophages. Our in vitro and ex vivo experimental data strongly suggest that AEBP1 plays critical regulatory roles in macrophage cholesterol homeostasis, foam cell formation, and proinflammation. Thereby, we speculate that AEBP1 may be critically implicated in the development of atherosclerosis, and it may serve as a molecular target toward developing antiinflammatory, antiatherogenic therapeutic approaches.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures



Similar articles
-
PPARgamma1 and LXRalpha face a new regulator of macrophage cholesterol homeostasis and inflammatory responsiveness, AEBP1.Nucl Recept Signal. 2010 Apr 16;8:e004. doi: 10.1621/nrs.08004. Nucl Recept Signal. 2010. PMID: 20419060 Free PMC article. Review.
-
Adipocyte enhancer-binding protein 1 (AEBP1) (a novel macrophage proinflammatory mediator) overexpression promotes and ablation attenuates atherosclerosis in ApoE (-/-) and LDLR (-/-) mice.Mol Med. 2011 Sep-Oct;17(9-10):1056-64. doi: 10.2119/molmed.2011.00141. Epub 2011 Jun 14. Mol Med. 2011. PMID: 21687917 Free PMC article.
-
LPS-induced suppression of macrophage cholesterol efflux is mediated by adipocyte enhancer-binding protein 1.Int J Biochem Cell Biol. 2009 Jul;41(7):1518-25. doi: 10.1016/j.biocel.2009.01.003. Epub 2009 Jan 8. Int J Biochem Cell Biol. 2009. PMID: 19166963
-
A growth hormone-releasing peptide that binds scavenger receptor CD36 and ghrelin receptor up-regulates sterol transporters and cholesterol efflux in macrophages through a peroxisome proliferator-activated receptor gamma-dependent pathway.Mol Endocrinol. 2006 Dec;20(12):3165-78. doi: 10.1210/me.2006-0146. Epub 2006 Sep 7. Mol Endocrinol. 2006. PMID: 16959872
-
Regulation of IkappaBalpha function and NF-kappaB signaling: AEBP1 is a novel proinflammatory mediator in macrophages.Mediators Inflamm. 2010;2010:823821. doi: 10.1155/2010/823821. Epub 2010 Apr 12. Mediators Inflamm. 2010. PMID: 20396415 Free PMC article. Review.
Cited by
-
Atlas of clinically distinct cell states and ecosystems across human solid tumors.Cell. 2021 Oct 14;184(21):5482-5496.e28. doi: 10.1016/j.cell.2021.09.014. Epub 2021 Sep 30. Cell. 2021. PMID: 34597583 Free PMC article.
-
AEBP1 is a negative regulator of skeletal muscle cell differentiation in oral squamous cell carcinoma.Sci Rep. 2024 Nov 9;14(1):27425. doi: 10.1038/s41598-024-79061-3. Sci Rep. 2024. PMID: 39521917 Free PMC article.
-
Carboxypeptidase-M is regulated by lipids and CSFs in macrophages and dendritic cells and expressed selectively in tissue granulomas and foam cells.Lab Invest. 2012 Mar;92(3):345-61. doi: 10.1038/labinvest.2011.168. Epub 2011 Dec 12. Lab Invest. 2012. PMID: 22157720 Free PMC article.
-
PPARgamma1 and LXRalpha face a new regulator of macrophage cholesterol homeostasis and inflammatory responsiveness, AEBP1.Nucl Recept Signal. 2010 Apr 16;8:e004. doi: 10.1621/nrs.08004. Nucl Recept Signal. 2010. PMID: 20419060 Free PMC article. Review.
-
Adipocyte enhancer-binding protein 1 (AEBP1) (a novel macrophage proinflammatory mediator) overexpression promotes and ablation attenuates atherosclerosis in ApoE (-/-) and LDLR (-/-) mice.Mol Med. 2011 Sep-Oct;17(9-10):1056-64. doi: 10.2119/molmed.2011.00141. Epub 2011 Jun 14. Mol Med. 2011. PMID: 21687917 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

