Cyclin D1b variant influences prostate cancer growth through aberrant androgen receptor regulation
- PMID: 16461912
- PMCID: PMC1413684
- DOI: 10.1073/pnas.0506281103
Cyclin D1b variant influences prostate cancer growth through aberrant androgen receptor regulation
Abstract
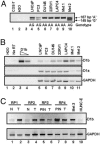
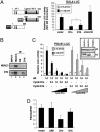
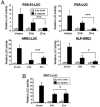
Cyclin D1 is a multifaceted regulator of both transcription and cell-cycle progression that exists in two distinct isoforms, cyclin D1a and D1b. In the prostate, cyclin D1a acts through discrete mechanisms to negatively regulate androgen receptor (AR) activity and thus limit androgen-dependent proliferation. Accordingly, cyclin D1a is rarely overexpressed in prostatic adenocarcinoma and holds little prognostic value in this tumor type. However, a common polymorphism (A870) known to facilitate production of cyclin D1b is associated with increased prostate cancer risk. Here we show that cyclin D1b is expressed at high frequency in prostate cancer and is up-regulated in neoplastic disease. Furthermore, our data demonstrate that, although cyclin D1b retains AR association, it is selectively compromised for AR regulation. The altered ability of cyclin D1b to regulate the AR was observed by using both in vitro and in vivo assays and was associated with compromised regulation of AR-dependent proliferation. Consistent with previous reports, expression of cyclin D1a inhibited cell-cycle progression in AR-dependent prostate cancer cells. Strikingly, cyclin D1b significantly stimulated proliferation in this cell type. AR-negative prostate cancer cells were nonresponsive to cyclin D1 (a or b) expression, indicating that defects in AR corepressor function yield a growth advantage specifically in AR-dependent cells. In summary, these studies indicate that the altered AR regulatory capacity of cyclin D1b contributes to its association with increased prostate cancer risk and provide evidence of cyclin D1b-mediated transcriptional regulation.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures
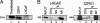


References
-
- Salesi N., Carlini P., Ruggeri E. M., Ferretti G., Bria E., Cognetti F. J. Exp. Clin. Cancer Res. 2005;24:175–180. - PubMed
-
- Feldman B. J., Feldman D. Nat. Rev. Cancer. 2001;1:34–45. - PubMed
-
- Trapman J., Brinkmann A. O. Pathol. Res. Pract. 1996;192:752–760. - PubMed
-
- He B., Kemppainen J. A., Wilson E. M. J. Biol. Chem. 2000;275:22986–22994. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32 ES007250/ES/NIEHS NIH HHS/United States
- CA106471/CA/NCI NIH HHS/United States
- R01 DK065264/DK/NIDDK NIH HHS/United States
- DK065264/DK/NIDDK NIH HHS/United States
- T32 CA117846/CA/NCI NIH HHS/United States
- P-30-ES06096/ES/NIEHS NIH HHS/United States
- P30 ES006096/ES/NIEHS NIH HHS/United States
- T32 ES07250-16/ES/NIEHS NIH HHS/United States
- R01 CA106471/CA/NCI NIH HHS/United States
- CA 093404/CA/NCI NIH HHS/United States
- R01 CA093404/CA/NCI NIH HHS/United States
- R01 CA099996/CA/NCI NIH HHS/United States
- CA 099996/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

