Mapping the interaction surface of linker histone H1(0) with the nucleosome of native chromatin in vivo
- PMID: 16462749
- PMCID: PMC1868459
- DOI: 10.1038/nsmb1050
Mapping the interaction surface of linker histone H1(0) with the nucleosome of native chromatin in vivo
Erratum in
- Nat Struct Mol Biol. 2006 May;13(5):465
Abstract
H1 linker histones stabilize the nucleosome, limit nucleosome mobility and facilitate the condensation of metazoan chromatin. Here, we have combined systematic mutagenesis, measurement of in vivo binding by photobleaching microscopy, and structural modeling to determine the binding geometry of the globular domain of the H1(0) linker histone variant within the nucleosome in unperturbed, native chromatin in vivo. We demonstrate the existence of two distinct DNA-binding sites within the globular domain that are formed by spatial clustering of multiple residues. The globular domain is positioned via interaction of one binding site with the major groove near the nucleosome dyad. The second site interacts with linker DNA adjacent to the nucleosome core. Multiple residues bind cooperatively to form a highly specific chromatosome structure that provides a mechanism by which individual domains of linker histones interact to facilitate chromatin condensation.
Figures



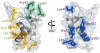

References
-
- Wolffe AP. Chromatin: Structure and Function. 3rd edn Academic Press; San Diego, USA: 1995.
-
- Noll M, Kornberg RD. Action of microccoccal nuclease on chromatin and the location of histone H1. J. Mol. Biol. 1977;109:393–404. - PubMed
-
- Simpson RT. Structure of the chromatosome, a chromatin particle containing 160 base pairs of DNA and all the histones. Biochemistry. 1978;17:5524–5531. - PubMed
-
- Ramakrishnan V. Histone H1 and chromatin higher order structure. Crit. Rev. Eukaryot. Gene Expr. 1997;7:215–230. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

