Highly active chiral ruthenium catalysts for asymmetric ring-closing olefin metathesis
- PMID: 16464082
- PMCID: PMC2533259
- DOI: 10.1021/ja055994d
Highly active chiral ruthenium catalysts for asymmetric ring-closing olefin metathesis
Abstract
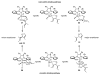
The synthesis of olefin metathesis catalysts containing chiral, monodentate N-heterocyclic carbenes and their application to asymmetric ring-closing metathesis (ARCM) are reported. These catalysts retain the high levels of reactivity found in the related achiral variants (1a and 1b). Using the parent chiral catalysts 2a and 2b and derivatives that contain steric bulk in the meta positions of the N-bound aryl rings (catalysts 3-5), five- through seven-membered rings were formed in up to 92% ee. The addition of sodium iodide to catalysts 2a-4a (to form 2b-4b in situ) caused a dramatic increase in enantioselectivity for many substrates. Catalyst 5a, which gave high enantiomeric excesses for certain substrates without the addition of NaI, could be used in loadings of < or =1 mol %. Mechanistic explanations for the large sodium iodide effect as well as possible mechanistic pathways leading to the observed products are discussed.
Figures
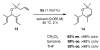
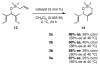
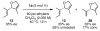
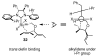


References
-
-
For recent reviews on olefin metathesis, see: Grubbs RH, editor. Handbook of Metathesis. Wiley-VCH; Weinheim: 2003. Trnka T, Grubbs RH. Acc Chem Res. 2001;34:18–29.Fürstner A. Angew Chem Int Ed. 2000;39:3012–3043.Grubbs RH, Chang S. Tetrahedron. 1998;54:4413–4450.Ivin KJ. J Mol Cat A Chem. 1998;133:1–16.
-
-
- Fujimura O, Grubbs RH. J Am Chem Soc. 1996;118:2499–2500.
- Fujimura O, de la Mata FJ, Grubbs RH. Organometallics. 1996;15:1865–1871.
-
-
Ruthenium catalysts: Van Veldhuizen JJ, Gillingham DG, Garber SB, Kataoka O, Hoveyda AH. J Am Chem Soc. 2003;125:12502–12508.Van Veldhuizen JJ, Garber SB, Kingsbury JS, Hoveyda AH. J Am Chem Soc. 2002;124:4954–4955.Seiders TJ, Ward DW, Grubbs RH. Org Lett. 2001;3:3225–3228.
-
-
-
Molybdenum catalysts: Totland KM, Boyd TJ, Lavoie GG, Davis WM, Schrock RR. Macromolecules. 1996;29:6114–6125.Alexander JB, La DS, Cefalo DR, Hoveyda AH, Schrock RR. J Am Chem Soc. 1998;120:4041–4042.Zhu SS, Cefalo DR, La DS, Jamieson JY, Davis WM, Hoveyda AH, Schrock RR. J Am Chem Soc. 1999;121:8251–8259.Alexander JB, Schrock RR, Davis WM, Hultzsch KC, Hoveyda AH, Houser JH. Organometallics. 2000;19:3700–3715.Aeilts SL, Cefalo DR, Bonitatebus PJ, Jr, Houser JH, Hoveyda AH, Schrock RR. Angew Chem Int Ed. 2001;40:1452–1456.Tsang WCP, Schrock RR, Hoveyda AH. Organometallics. 2001;20:5658–5669.Hultzsch KC, Bonitatebus PJ, Jernelius J, Schrock RR, Hoveyda AH. Organometallics. 2001;20:4705–4712.Schrock RR, Jamieson JY, Dolman SJ, Miller SA, Bonitatebus PJ, Hoveyda AH. Organometallics. 2002;21:409–417.Tsang WCP, Jernelius JA, Cortez GA, Weatherhead GS, Schrock RR, Hoveyda AH. J Am Chem Soc. 2003;125:2591–2596.
-
-
- Sattely ES, Cortez GA, Moebius DC, Schrock RR, Hoveyda AH. J Am Chem Soc. 2005;127:8526–8533. - PubMed
- Jernelius JA, Schrock RR, Hoveyda AH. Tetrahedron. 2004;60:7345–7351.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

