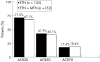Efficacy and safety of combination etanercept and methotrexate versus etanercept alone in patients with rheumatoid arthritis with an inadequate response to methotrexate: the ADORE study
- PMID: 16464988
- PMCID: PMC1798368
- DOI: 10.1136/ard.2005.043299
Efficacy and safety of combination etanercept and methotrexate versus etanercept alone in patients with rheumatoid arthritis with an inadequate response to methotrexate: the ADORE study
Abstract
Objective: To evaluate the efficacy and safety of etanercept (ETN) monotherapy compared with combination ETN and methotrexate (MTX) treatment in patients with rheumatoid arthritis who had an inadequate response to MTX monotherapy. (The response was defined by the presence of Disease Activity Score-28 joint count (DAS28) >or=3.2 or a combination of >or=5 swollen joints, >or=5 painful joints and erythrocyte sedimentation rate >or=10 mm/h.)
Methods: Patients with active rheumatoid arthritis taking MTX >or=12.5 mg/week for >or=3 months were included in this 16 week, randomised, open-label study. Patients were randomly assigned to either ETN (25 mg subcutaneous injection twice weekly) added to the baseline dose of MTX or ETN monotherapy.
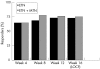
Results: 315 patients were randomised to ETN (n = 160) or ETN plus MTX (n = 155). The primary end point, DAS28 (4) improvement of >1.2 units, was achieved by 72.8% and 75.2% of patients treated with ETN and those treated with ETN plus MTX, respectively, with no significant difference (p = 0.658) between the two groups. The European League Against Rheumatism response criteria of good or moderate response was attained by 80.0% of patients in the ETN group and by 82.4% of patients in the ETN plus MTX group. American College of Rheumatology 20%, 50% and 70% response rates achieved by both groups were also similar: 71.0% v 67.1%, 41.9% v 40.1% and 17.4% v 18.4%, respectively. The rates of adverse and serious adverse events were similar between the treatment groups.
Conclusion: Both the addition of ETN to MTX and the substitution of ETN for MTX in patients with rheumatoid arthritis who had an inadequate response to MTX resulted in substantial improvements in clinical signs and symptoms and were generally well-tolerated treatment strategies for improving clinical signs and symptoms of rheumatoid arthritis.
Conflict of interest statement
Competing interests: None declared.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous