N-arachidonoyl L-serine, an endocannabinoid-like brain constituent with vasodilatory properties
- PMID: 16467152
- PMCID: PMC1413724
- DOI: 10.1073/pnas.0510676103
N-arachidonoyl L-serine, an endocannabinoid-like brain constituent with vasodilatory properties
Abstract
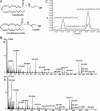
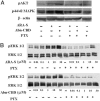
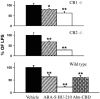
The endocannabinoid N-arachidonoyl ethanolamine (anandamide), found both in the CNS and in the periphery, plays a role in numerous physiological systems. One might expect that the chemically related N-arachidonoyl-L-serine (ARA-S) could also be formed alongside anandamide. We have now isolated ARA-S from bovine brain and elucidated its structure by comparison with synthetic ARA-S. Contrary to anandamide, ARA-S binds very weakly to cannabinoid CB1 and CB2 or vanilloid TRPV1 (transient receptor potential vanilloid 1) receptors. However, it produces endothelium-dependent vasodilation of rat isolated mesenteric arteries and abdominal aorta and stimulates phosphorylation of p44/42 mitogen-activated protein (MAP) kinase and protein kinase B/Akt in cultured endothelial cells. ARA-S also suppresses LPS-induced formation of TNF-alpha in a murine macrophage cell line and in wild-type mice, as well as in mice deficient in CB1 or CB2 receptors. Many of these effects parallel those reported for abnormal cannabidiol (Abn-CBD), a synthetic agonist of a putative novel cannabinoid-type receptor. Hence, ARA-S may represent an endogenous agonist for this receptor.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures

References
-
- Gaoni Y., Mechoulam R. J. Am. Chem. Soc. 1964;86:1646–1647.
-
- Iversen L. L. The Science of Marijuana. New York: Oxford Univ. Press; 2000.
-
- Devane W. A., Dysarz F. A., Johnson M. R., Melvin L. S., Howlett A. C. Mol. Pharmacol. 1988;34:605–613. - PubMed
-
- Munro S., Thomas K. L., Abu-Shaar M. Nature. 1993;365:61–65. - PubMed
-
- Matsuda L. A., Lolait S. J., Brownstein M. J., Young A. C., Bonner T. I. Nature. 1990;346:561–564. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

