The Saccharomyces cerevisiae Lipin homolog is a Mg2+-dependent phosphatidate phosphatase enzyme
- PMID: 16467296
- PMCID: PMC1424669
- DOI: 10.1074/jbc.M600425200
The Saccharomyces cerevisiae Lipin homolog is a Mg2+-dependent phosphatidate phosphatase enzyme
Abstract
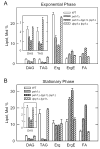
Mg(2+)-dependent phosphatidate (PA) phosphatase (3-sn-phosphatidate phosphohydrolase, EC 3.1.3.4) catalyzes the dephosphorylation of PA to yield diacylglycerol and P(i). In this work, we identified the Saccharomyces cerevisiae PAH1 (previously known as SMP2) gene that encodes Mg(2+)-dependent PA phosphatase using amino acid sequence information derived from a purified preparation of the enzyme (Lin, Y.-P., and Carman, G. M. (1989) J. Biol. Chem. 264, 8641-8645). Overexpression of PAH1 in S. cerevisiae directed elevated levels of Mg(2+)-dependent PA phosphatase activity, whereas the pah1Delta mutation caused reduced levels of enzyme activity. Heterologous expression of PAH1 in Escherichia coli confirmed that Pah1p is a Mg(2+)-dependent PA phosphatase enzyme and showed that its enzymological properties were very similar to those of the enzyme purified from S. cerevisiae. The PAH1-encoded enzyme activity was associated with both the membrane and cytosolic fractions of the cell, and the membrane-bound form of the enzyme was salt-extractable. Lipid analysis showed that mutants lacking PAH1 accumulated PA and had reduced amounts of diacylglycerol and its derivative triacylglycerol.ThePAH1-encoded Mg(2+)-dependent PA phosphatase shows homology to mammalian lipin, a fat-regulating protein whose molecular function is unknown. Heterologous expression of human LPIN1 in E. coli showed that lipin 1 is also a Mg(2+)-dependent PA phosphatase enzyme.
Figures




References
-
- Smith SW, Weiss SB, Kennedy EP. J Biol Chem. 1957;228:915–922. - PubMed
-
- Carman GM, Henry SA. Annu Rev Biochem. 1989;58:635–669. - PubMed
-
- Carman GM, Zeimetz GM. J Biol Chem. 1996;271:13293–13296. - PubMed
-
- Henry SA, Patton-Vogt JL. Prog Nucleic Acid Res. 1998;61:133–179. - PubMed
-
- Carman GM, Henry SA. Prog Lipid Res. 1999;38:361–399. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

