Kinesin-1 and Dynein are the primary motors for fast transport of mitochondria in Drosophila motor axons
- PMID: 16467387
- PMCID: PMC1415296
- DOI: 10.1091/mbc.e05-06-0526
Kinesin-1 and Dynein are the primary motors for fast transport of mitochondria in Drosophila motor axons
Abstract
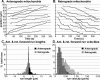
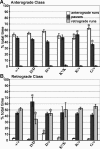
To address questions about mechanisms of filament-based organelle transport, a system was developed to image and track mitochondria in an intact Drosophila nervous system. Mutant analyses suggest that the primary motors for mitochondrial movement in larval motor axons are kinesin-1 (anterograde) and cytoplasmic dynein (retrograde), and interestingly that kinesin-1 is critical for retrograde transport by dynein. During transport, there was little evidence that force production by the two opposing motors was competitive, suggesting a mechanism for alternate coordination. Tests of the possible coordination factor P150(Glued) suggested that it indeed influenced both motors on axonal mitochondria, but there was no evidence that its function was critical for the motor coordination mechanism. Observation of organelle-filled axonal swellings ("organelle jams" or "clogs") caused by kinesin and dynein mutations showed that mitochondria could move vigorously within and pass through them, indicating that they were not the simple steric transport blockades suggested previously. We speculate that axonal swellings may instead reflect sites of autophagocytosis of senescent mitochondria that are stranded in axons by retrograde transport failure; a protective process aimed at suppressing cell death signals and neurodegeneration.
Figures






References
-
- Bowman, A. B., Patel-King, R. S., Benashski, S. E., McCaffery, J. M., Goldstein, L.S.B., and King, S. M. (1999). Drosophila roadblock and Chlamydomonas LC7: a conserved family of dynein-associated proteins involved in axonal transport, flagellar motility, and mitosis. J. Cell Biol. 146, 165-180. - PMC - PubMed
-
- Brand, A. H., and Perrimon, N. (1993). Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118, 401-415. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

