Direct GABAergic and glycinergic inhibition of the substantia gelatinosa from the rostral ventromedial medulla revealed by in vivo patch-clamp analysis in rats
- PMID: 16467527
- PMCID: PMC6793630
- DOI: 10.1523/JNEUROSCI.4856-05.2006
Direct GABAergic and glycinergic inhibition of the substantia gelatinosa from the rostral ventromedial medulla revealed by in vivo patch-clamp analysis in rats
Abstract
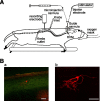
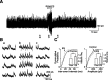
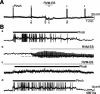
Stimulation of the rostral ventromedial medulla (RVM) is believed to exert analgesic effects through the activation of the serotonergic system descending to the spinal dorsal horn; however, how nociceptive transmission is modulated by the descending system has not been fully clarified. To investigate the inhibitory mechanisms affected by the RVM, an in vivo patch-clamp technique was used to record IPSCs from the substantia gelatinosa (SG) of the spinal cord evoked by chemical (glutamate injection) and electrical stimulation (ES) of the RVM in adult rats. In the voltage-clamp mode, the RVM glutamate injection and RVM-ES produced an increase in both the frequency and amplitude of IPSCs in SG neurons that was not blocked by glutamate receptor antagonists. Serotonin receptor antagonists were unexpectedly without effect, but a GABAA receptor antagonist, bicuculline, or a glycine receptor antagonist, strychnine, completely suppressed the RVM stimulation-induced increase in IPSCs. The RVM-ES-evoked IPSCs showed fixed latency and no failure at 20 Hz stimuli with a conduction velocity of >3 m/s (3.1-20.7 m/s), suggesting descending monosynaptic GABAergic and/or glycinergic inputs from the RVM to the SG through myelinated fibers. In the current-clamp mode, action potentials elicited by noxious mechanical stimuli applied to the receptive field of the ipsilateral hindlimb were suppressed by the RVM-ES in more than half of the neurons tested (63%; 10 of 16). These findings suggest that the RVM-mediated antinociceptive effects on noxious inputs to the SG may be exerted preferentially by the direct GABAergic and glycinergic pathways to the SG.
Figures





Similar articles
-
Primary afferent-evoked glycine- and GABA-mediated IPSPs in substantia gelatinosa neurones in the rat spinal cord in vitro.J Physiol. 1995 Jan 1;482 ( Pt 1)(Pt 1):29-38. doi: 10.1113/jphysiol.1995.sp020497. J Physiol. 1995. PMID: 7730987 Free PMC article.
-
In vivo patch-clamp analysis of IPSCs evoked in rat substantia gelatinosa neurons by cutaneous mechanical stimulation.J Neurophysiol. 2000 Oct;84(4):2171-4. doi: 10.1152/jn.2000.84.4.2171. J Neurophysiol. 2000. PMID: 11024105
-
Actions of propofol on substantia gelatinosa neurones in rat spinal cord revealed by in vitro and in vivo patch-clamp recordings.Eur J Neurosci. 2009 Feb;29(3):518-28. doi: 10.1111/j.1460-9568.2008.06607.x. Eur J Neurosci. 2009. PMID: 19222560
-
GABA(B) receptor-mediated presynaptic inhibition of glycinergic transmission onto substantia gelatinosa neurons in the rat spinal cord.Pain. 2008 Aug 31;138(2):330-342. doi: 10.1016/j.pain.2008.01.005. Epub 2008 Feb 6. Pain. 2008. PMID: 18258370
-
Phospholipase A2 activation enhances inhibitory synaptic transmission in rat substantia gelatinosa neurons.J Neurophysiol. 2008 Mar;99(3):1274-84. doi: 10.1152/jn.01292.2007. Epub 2008 Jan 23. J Neurophysiol. 2008. PMID: 18216222
Cited by
-
Differential wiring of local excitatory and inhibitory synaptic inputs to islet cells in rat spinal lamina II demonstrated by laser scanning photostimulation.J Physiol. 2007 May 1;580(Pt.3):815-33. doi: 10.1113/jphysiol.2007.128314. Epub 2007 Feb 8. J Physiol. 2007. PMID: 17289782 Free PMC article.
-
In vivo patch-clamp analysis of response properties of rat primary somatosensory cortical neurons responding to noxious stimulation of the facial skin.Mol Pain. 2010 May 26;6:30. doi: 10.1186/1744-8069-6-30. Mol Pain. 2010. PMID: 20500889 Free PMC article.
-
Postnatal development shifts the balance of pain descending control.J Physiol. 2009 Jun 15;587(Pt 12):2711-2. doi: 10.1113/jphysiol.2009.174565. J Physiol. 2009. PMID: 19525553 Free PMC article. No abstract available.
-
Systemic dexmedetomidine augments inhibitory synaptic transmission in the superficial dorsal horn through activation of descending noradrenergic control: an in vivo patch-clamp analysis of analgesic mechanisms.Pain. 2014 Mar;155(3):617-628. doi: 10.1016/j.pain.2013.12.018. Epub 2013 Dec 16. Pain. 2014. PMID: 24355412 Free PMC article.
-
Spinal Circuits Transmitting Mechanical Pain and Itch.Neurosci Bull. 2018 Feb;34(1):186-193. doi: 10.1007/s12264-017-0136-z. Epub 2017 May 8. Neurosci Bull. 2018. PMID: 28484964 Free PMC article. Review.
References
-
- Antal M, Petko M, Polgar E, Heizmann CW, Storm-Mathisen J (1996). Direct evidence of an extensive GABAergic innervation of the spinal dorsal horn by fibres descending from the rostral ventromedial medulla. Neuroscience 73:509–518. - PubMed
-
- Azami J, Llewelyn MB, Roberts MH (1982). The contribution of nucleus reticularis paragigantocellularis and nucleus raphe magnus to the analgesia produced by systemically administered morphine, investigated with the microinjection technique. Pain 12:229–246. - PubMed
-
- Basbaum AI, Zahs K, Lord B, Lakos S (1988). The fiber caliber of 5-HT immunoreactive axons in the dorsolateral funiculus of the spinal cord of the rat and cat. Somatosens Res 5:177–185. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
