Loss of leukemia inhibitory factor receptor beta or cardiotrophin-1 causes similar deficits in preganglionic sympathetic neurons and adrenal medulla
- PMID: 16467531
- PMCID: PMC6793615
- DOI: 10.1523/JNEUROSCI.4127-05.2006
Loss of leukemia inhibitory factor receptor beta or cardiotrophin-1 causes similar deficits in preganglionic sympathetic neurons and adrenal medulla
Abstract
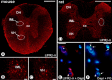
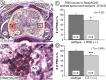
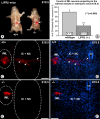
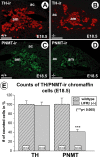
Leukemia inhibitory factor (LIF) receptor beta (LIFRbeta) is a receptor for a variety of neurotrophic cytokines, including LIF, ciliary neurotrophic factor (CNTF), and cardiotrophin-1 (CT-1). These cytokines play an essential role for the survival and maintenance of developing and postnatal somatic motoneurons. CNTF may also serve the maintenance of autonomic, preganglionic sympathetic neurons (PSNs) in the spinal cord, as suggested by its capacity to prevent their death after destruction of one of their major targets, the adrenal medulla. Although somatic motoneurons and PSNs share a common embryonic origin, they are distinct in several respects, including responses to lesions. We have studied PSNs in mice with targeted deletions of the LIFRbeta or CT-1 genes, respectively. We show that LIF, CNTF, and CT-1 are synthesized in embryonic adrenal gland and spinal cord and that PSNs express LIFRbeta. In embryonic day 18.5 LIFRbeta (-/-) and CT-1 (-/-) mice, PSNs were reduced by approximately 20%. PSNs projecting to the adrenal medulla were more severely affected (-55%). Although LIFRbeta (-/-) mice revealed normal numbers of adrenal chromaffin cells and axons terminating on chromaffin cells, levels of adrenaline and numbers of adrenaline-synthesizing cells were significantly reduced. We conclude that activation of LIFRbeta is required for normal development of PSNs and one of their prominent targets, the adrenal medulla. Thus, both somatic motoneurons and PSNs in the spinal cord depend on LIFRbeta signaling for their development and maintenance, although PSNs seem to be overall less affected than somatic motoneurons by LIFRbeta deprivation.
Figures



Similar articles
-
Triple knock-out of CNTF, LIF, and CT-1 defines cooperative and distinct roles of these neurotrophic factors for motoneuron maintenance and function.J Neurosci. 2005 Feb 16;25(7):1778-87. doi: 10.1523/JNEUROSCI.4249-04.2005. J Neurosci. 2005. PMID: 15716414 Free PMC article.
-
Cardiotrophin-1, a muscle-derived cytokine, is required for the survival of subpopulations of developing motoneurons.J Neurosci. 2001 Feb 15;21(4):1283-91. doi: 10.1523/JNEUROSCI.21-04-01283.2001. J Neurosci. 2001. PMID: 11160399 Free PMC article.
-
Cardiotrophin-1 requires LIFRbeta to promote survival of mouse motoneurons purified by a novel technique.J Neurosci Res. 1999 Jan 1;55(1):119-26. doi: 10.1002/(SICI)1097-4547(19990101)55:1<119::AID-JNR13>3.0.CO;2-6. J Neurosci Res. 1999. PMID: 9890440
-
Growth and neurotrophic factors regulating development and maintenance of sympathetic preganglionic neurons.Int Rev Cytol. 2001;205:37-76. doi: 10.1016/s0074-7696(01)05002-1. Int Rev Cytol. 2001. PMID: 11336393 Review.
-
Selective destruction of preganglionic sympathetic nerves by antibodies to acetylcholinesterase.J Neural Transm Suppl. 1991;34:139-45. doi: 10.1007/978-3-7091-9175-0_18. J Neural Transm Suppl. 1991. PMID: 1817157 Review.
Cited by
-
Progressive postnatal motoneuron loss in mice lacking GDF-15.J Neurosci. 2009 Oct 28;29(43):13640-8. doi: 10.1523/JNEUROSCI.1133-09.2009. J Neurosci. 2009. PMID: 19864576 Free PMC article.
-
Transforming growth factor-{beta} coordinately induces suppressor of cytokine signaling 3 and leukemia inhibitory factor to suppress osteoclast apoptosis.Endocrinology. 2010 Apr;151(4):1713-22. doi: 10.1210/en.2009-0813. Epub 2010 Feb 24. Endocrinology. 2010. PMID: 20181800 Free PMC article.
-
Stüve-Wiedemann syndrome: LIFR and associated cytokines in clinical course and etiology.Orphanet J Rare Dis. 2014 Mar 12;9:34. doi: 10.1186/1750-1172-9-34. Orphanet J Rare Dis. 2014. PMID: 24618404 Free PMC article. Review.
-
Mechanism of shortened bones in mucopolysaccharidosis VII.Mol Genet Metab. 2009 Jul;97(3):202-11. doi: 10.1016/j.ymgme.2009.03.005. Epub 2009 Mar 25. Mol Genet Metab. 2009. PMID: 19375967 Free PMC article.
-
ApoER2 and VLDLr are required for mediating reelin signalling pathway for normal migration and positioning of mesencephalic dopaminergic neurons.PLoS One. 2013 Aug 16;8(8):e71091. doi: 10.1371/journal.pone.0071091. eCollection 2013. PLoS One. 2013. PMID: 23976984 Free PMC article.
References
-
- Andrä J, Lojda Z (1986). A histochemical method for the demonstration of acetylcholinesterase activity using semipermeable membranes. Histochemistry 84:575–579. - PubMed
-
- Bazan JF (1991). Neuropoietic cytokines in the hematopoietic fold. Neuron 7:197–208. - PubMed
-
- Blottner D, Baumgarten H-G (1992). Nitric oxides synthas (NOS)-containing sympathoadrenal cholinergic neurons of the rat IML-cell column: evidence from histochemistry, and retrograde labeling. J Comp Neurol 316:45–55. - PubMed
-
- Blottner D, Unsicker K (1990). Maintenance of intermediolateral spinal cord neurons by fibroblast growth factor administered to the medullectomized rat adrenal gland: dependence on intact organ innervation and cellular organization of implants. Eur J Neurosci 2:378–382. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
