Cell-specific repressor or enhancer activities of Deaf-1 at a serotonin 1A receptor gene polymorphism
- PMID: 16467535
- PMCID: PMC6793620
- DOI: 10.1523/JNEUROSCI.2643-05.2006
Cell-specific repressor or enhancer activities of Deaf-1 at a serotonin 1A receptor gene polymorphism
Abstract
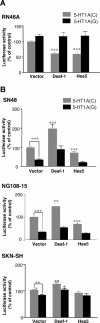
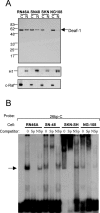
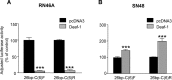
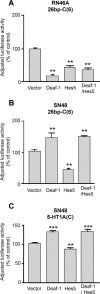
The serotonin-1A (5-HT1A) receptor is the primary somatodendritic autoreceptor that inhibits the activity of serotonergic raphe neurons and is also expressed in nonserotonergic cortical and limbic neurons. Alterations in 5-HT1A receptor levels are implicated in mood disorders, and a functional C(-1019)G 5-HT1A promoter polymorphism has been associated with depression, suicide, and panic disorder. We examined the cell-specific activity of identified transcription factors, human nuclear deformed epidermal autoregulatory factor-1 (DEAF-1)-related (NUDR)/Deaf-1 and Hes5, at the 5-HT1A C(-1019) site. In serotonergic raphe RN46A cells, Deaf-1 and Hes5 repressed the 5-HT1A receptor gene at the C(-1019)-allele but not the G(-1019)-allele. However, in nonserotonergic cells that express 5-HT1A receptors (septal SN48, neuroblastoma SKN-SH, and neuroblastoma/glioma NG108-15 cells), Deaf-1 enhanced 5-HT1A promoter activity at the C(-1019)-allele but not the G-allele, whereas Hes5 repressed in all cell types. The enhancer activity of Deaf-1 was orientation independent and competed out Hes5 repression. To test whether Deaf-1 activity is intrinsic, the activity of a Gal4DBD (DNA binding domain)-Deaf-1 fusion protein at a heterologous Gal4 DNA element was examined. Gal4DBD-Deaf-1 repressed transcription in RN46A cells but enhanced transcription in SN48 cells, indicating that these opposite activities are intrinsic to Deaf-1. Repressor or enhancer activities of Deaf-1 or Gal4DBD-Deaf-1 were blocked by histone deacetylase inhibitor trichostatin A. Thus, the intrinsic activity of Deaf-1 at the 5-HT1A promoter is opposite in presynaptic versus postsynaptic neuronal cells and requires deacetylation. Cell-specific regulation by Deaf-1 could underlie region-specific alterations in 5-HT1A receptor expression in different mood disorders.
Figures


References
-
- Albert PR, Lemonde S (2004). 5-HT1A receptors, gene repression, and depression: guilt by association. Neuroscientist 10:575–593. - PubMed
-
- Ansorge M, Tanneberger C, Davies B, Theuring F, Kusserow H (2004). Analysis of the murine 5-HT receptor gene promoter in vitro and in vivo. Eur J Neurosci 20:363–374. - PubMed
-
- Ayer DE (1999). Histone deacetylases: transcriptional repression with SINers and NuRDs. Trends Cell Biol 9:193–198. - PubMed
-
- Bach I, Carriere C, Ostendorff HP, Andersen B, Rosenfeld MG (1997). A family of LIM domain-associated cofactors confer transcriptional synergism between LIM and Otx homeodomain proteins. Genes Dev 11:1370–1380. - PubMed
-
- Bayliss DA, Li YW, Talley EM (1997). Effects of serotonin on caudal raphe neurons: activation of an inwardly rectifying potassium conductance. J Neurophysiol 77:1349–1361. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
