Nanomedicine and protein misfolding diseases
- PMID: 16467913
- PMCID: PMC1351038
- DOI: 10.1016/j.nano.2005.10.005
Nanomedicine and protein misfolding diseases
Abstract
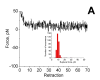
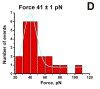
Misfolding and self assembly of proteins in nano-aggregates of different sizes and morphologies (nano-ensembles, primarily nanofilaments and nano-rings) is a complex phenomenon that can be facilitated, impeded, or prevented, by interactions with various intracellular metabolites, intracellular nanomachines controlling protein folding and interactions with other proteins. A fundamental understanding of molecular processes leading to misfolding and self-aggregation of proteins involved in various neurodegenerative diseases will provide critical information to help identify appropriate therapeutic routes to control these processes. An elevated propensity of misfolded protein conformation in solution to aggregate with the formation of various morphologies impedes the use of traditional physical chemical approaches for studies of misfolded conformations of proteins. In our recent alternative approach, the protein molecules were tethered to surfaces to prevent aggregation and AFM force spectroscopy was used to probe the interaction between protein molecules depending on their conformations. It was shown that formation of filamentous aggregates is facilitated at pH values corresponding to the maximum of rupture forces. In this paper, a novel surface chemistry was developed for anchoring of amyloid beta (Abeta) peptides at their N-terminal moieties. The use of the site specific immobilization procedure allowed to measure the rupture of Abeta-Abeta contacts at single molecule level. The rupture of these contacts is accompanied by the extension of the peptide chain detected by a characteristic elasto-mechanical component of the force-distance curves. Potential applications of the nanomechanical studies to understanding the mechanisms of development of protein misfolding diseases are discussed.
Figures




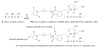
References
-
- Dobson CM. Principles of protein folding, misfolding and aggregation. Semin Cell Dev Biol. 2004;15:3–16. - PubMed
-
- Fink AL. Protein aggregation: folding aggregates, inclusion bodies and amyloid. Fold Des. 1998;3:R9–23. - PubMed
-
- Demidov VV. Proper Refolding helps express “difficult” proteins. Drug Discovery and Development. 2004;7:41–47.
-
- Ptitsyn OB. Molten globule and protein folding. Adv Protein Chem. 1995;47:83–229. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

