Tumor progression despite massive influx of activated CD8(+) T cells in a patient with malignant melanoma ascites
- PMID: 16468035
- PMCID: PMC11030266
- DOI: 10.1007/s00262-005-0118-2
Tumor progression despite massive influx of activated CD8(+) T cells in a patient with malignant melanoma ascites
Abstract
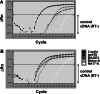
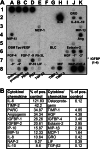
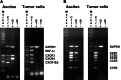
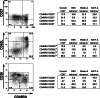
Although melanoma tumors usually express antigens that can be recognized by T cells, immune-mediated tumor rejection is rare. In many cases this is despite the presence of high frequencies of circulating tumor antigen-specific T cells, suggesting that tumor resistance downstream from T cell priming represents a critical barrier. Analyzing T cells directly from the melanoma tumor microenvironment, as well as the nature of the microenvironment itself, is central for understanding the key downstream mechanisms of tumor escape. In the current report we have studied tumor-associated lymphocytes from a patient with metastatic melanoma and large volume malignant ascites. The ascites fluid showed abundant tumor cells that expressed common melanoma antigens and retained expression of class I MHC and antigen processing machinery. The ascites fluid contained the chemokines CCL10, CCL15, and CCL18 which was associated with a large influx of activated T cells, including CD8(+) T cells recognizing HLA-A2 tetramer complexes with peptides from Melan-A and NA17-A. However, several functional defects of these tumor antigen-specific T cells were seen, including poor production of IFN-gamma in response to peptide-pulsed APC or autologous tumor cells, and lack of expression of perforin. Although these defects were T cell intrinsic, we also observed abundant CD4(+)CD25(+)FoxP3(+) T cells, as well as transcripts for FoxP3, IL-10, PD-L1/B7-H1, and indoleamine-2,3-dioxygenase (IDO). Our observations suggest that, despite recruitment of large numbers of activated CD8(+) T cells into the tumor microenvironment, T cell hyporesponsiveness and additional negative regulatory mechanisms can limit the effector phase of the anti-tumor immune response.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

