Correlation of F0F1-ATPase inhibition and antiproliferative activity of apoptolidin analogues
- PMID: 16468718
- PMCID: PMC2533578
- DOI: 10.1021/ol052800q
Correlation of F0F1-ATPase inhibition and antiproliferative activity of apoptolidin analogues
Abstract
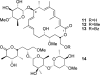
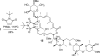
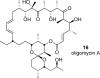
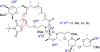
[structure: see text] Apoptolidin (1) exhibits potent and highly selective apoptosis inducing activity against sensitive cancer cell lines and is hypothesized to act by inhibition of mitochondrial F(0)F(1)-ATP synthase. A series of apoptolidin derivatives, including a new intermolecular Diels-Alder adduct, were analyzed for antiproliferative activity in E1A-transformed rat fibroblasts. Potent F(0)F(1)-ATPase inhibition was not a sufficient determinant of antiproliferative activity for several analogues, suggesting the existence of a secondary biological target or more complex mode of action for apoptolidin.
Figures


References
-
- Kim JW, Adachi H, Shin-ya K, Hayakawa Y, Seto H. J. Antibiotics. 1997;50:628. - PubMed
- Hayakawa Y, Kim JW, Adachi H, Shin-ya K, Fujita K, Seto H. J. Am. Chem Soc. 1998;120:3524.
-
- Wehlan H, Dauber M, Fernaud MTM, Schuppan J, Mahrwald R, Ziemer B, Garcia MEJ, Koert U. Angew. Chem. Int. Ed. 2004;43:2597. - PubMed
- Nicolaou KC, Fylaktakidou KC, Monenschein H, Li YW, Weyershausen B, Mitchell HJ, Wei HX, Guntupalli P, Hepworth D, Sugita K. J. Am. Chem. Soc. 2003;125:15433. - PubMed
- Nicolaou KC, Li YW, Sugita K, Monenschein H, Guntupalli P, Mitchell HJ, Fylaktakidou KC, Vourloumis D, Giannakakou P, O'Brate A. J. Am. Chem. Soc. 2003;125:15443. - PubMed
-
- Bouchez LC, Vogel P. Chem. Eur. J. 2005;11:4609. - PubMed
- Crimmins MT, Christie HS, Chaudhary K, Long A. J. Am. Chem. Soc. 2005;127:13810. - PubMed
- Jin B, Liu Q, Sulikowski GA. Tetrahedron. 2005;61:401.
- Wu B, Liu QS, Sulikowski GA. Angew. Chem. Int. Ed. 2004:6673–6675. - PubMed
- Abe K, Kato K, Arai T, Rahim MA, Sultana I, Matsumura S, Toshima K. Tetrahedron Lett. 2004;45:8849.
- Paquette WD, Taylor RE. Org. Lett. 2004;6:103. - PubMed
- Chng SS, Xu J, Loh TP. Tetrahedron Lett. 2003;44:4997.
- Chen Y, Evarts JB, Torres E, Fuchs PL. Org. Lett. 2002;4:3571. - PubMed
-
- Wender PA, Jankowski OD, Tabet EA, Seto H. Org. Lett. 2003;5:487. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

