Chelatases: distort to select?
- PMID: 16469498
- PMCID: PMC2997100
- DOI: 10.1016/j.tibs.2006.01.001
Chelatases: distort to select?
Abstract
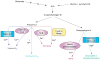
Chelatases catalyze the insertion of a specific metal ion into porphyrins, a key step in the synthesis of metalated tetrapyrroles that are essential for many cellular processes. Despite apparent common structural features among chelatases, no general reaction mechanism accounting for metal ion specificity has been established. We propose that chelatase-induced distortion of the porphyrin substrate not only enhances the reaction rate by decreasing the activation energy of the reaction but also modulates which divalent metal ion is incorporated into the porphyrin ring. We evaluate the recently recognized interaction between ferrochelatase and frataxin as a way to regulate iron delivery to ferrochelatase, and thus iron and heme metabolism. We postulate that the ferrochelatase-frataxin interaction controls the type of metal ion that is delivered to ferrochelatase.
Figures
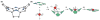


References
-
- Dioum EM, et al. NPAS2: a gas-responsive transcription factor. Science. 2002;298:2385–2387. - PubMed
-
- Kaasik K, Lee CC. Reciprocal regulation of haem biosynthesis and the circadian clock in mammals. Nature. 2004;430:467–471. - PubMed
-
- Rodgers KR. Heme-based sensors in biological systems. Curr Opin Chem Biol. 1999;3:158–167. - PubMed
-
- Al-Karadaghi S, et al. Crystal structure of ferrochelatase: the terminal enzyme in heme biosynthesis. Structure. 1997;5:1501–1510. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

