DEMETER DNA glycosylase establishes MEDEA polycomb gene self-imprinting by allele-specific demethylation
- PMID: 16469697
- PMCID: PMC4106368
- DOI: 10.1016/j.cell.2005.12.034
DEMETER DNA glycosylase establishes MEDEA polycomb gene self-imprinting by allele-specific demethylation
Abstract
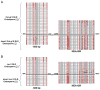
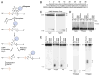
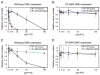
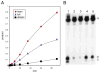
MEDEA (MEA) is an Arabidopsis Polycomb group gene that is imprinted in the endosperm. The maternal allele is expressed and the paternal allele is silent. MEA is controlled by DEMETER (DME), a DNA glycosylase required to activate MEA expression, and METHYLTRANSFERASE I (MET1), which maintains CG methylation at the MEA locus. Here we show that DME is responsible for endosperm maternal-allele-specific hypomethylation at the MEA gene. DME can excise 5-methylcytosine in vitro and when expressed in E. coli. Abasic sites opposite 5-methylcytosine inhibit DME activity and might prevent DME from generating double-stranded DNA breaks. Unexpectedly, paternal-allele silencing is not controlled by DNA methylation. Rather, Polycomb group proteins that are expressed from the maternal genome, including MEA, control paternal MEA silencing. Thus, DME establishes MEA imprinting by removing 5-methylcytosine to activate the maternal allele. MEA imprinting is subsequently maintained in the endosperm by maternal MEA silencing the paternal allele.
Figures


Comment in
-
MEDEA takes control of its own imprinting.Cell. 2006 Feb 10;124(3):468-70. doi: 10.1016/j.cell.2006.01.020. Cell. 2006. PMID: 16469694
References
-
- Bastow R, Mylne JS, Lister C, Lippman Z, Martienssen RA, Dean C. Vernalization requires epigenetic silencing of FLC by histone methylation. Nature. 2004;427:164–167. - PubMed
-
- Bender J. DNA methylation and epigenetics. Annu Rev Plant Biol. 2004;55:41–68. - PubMed
-
- Bhagwat M, Gerlt JA. 3′- and 5′-strand cleavage reactions catalyzed by the Fpg protein from Escherichia coli occur via successive β- and δ-elimination mechanisms, respectively. Biochemistry. 1996;35:659–665. - PubMed
-
- Cao R, Zhang Y. The functions of E(Z)/EZH2-mediated methylation of lysine 27 in histone H3. Curr Opin Genet Dev. 2004;14:155–164. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

