The triage of damaged proteins: degradation by the ubiquitin-proteasome pathway or repair by molecular chaperones
- PMID: 16469848
- PMCID: PMC2100384
- DOI: 10.1096/fj.05-5080fje
The triage of damaged proteins: degradation by the ubiquitin-proteasome pathway or repair by molecular chaperones
Abstract
Accumulation of damaged proteins is causally related to many age-related diseases. The ubiquitin-proteasome pathway (UPP) plays a role in selective degradation of damaged proteins, whereas molecular chaperones, such as heat shock proteins, are involved in refolding denatured proteins. This work demonstrates for the first time that the UPP and molecular chaperones work in a competitive manner and that the fates of denatured proteins are determined by the relative activities of the UPP and molecular chaperones. Enhanced UPP activity suppresses the refolding of denatured proteins whereas elevated chaperone activity inhibits the degradation of denatured proteins. CHIP, a co-chaperone with E3 activity, plays a pivotal role in determining the fates of the damaged proteins. The delicate balance between UPP-mediated degradation and refolding of denatured proteins is governed by relative levels of CHIP and other molecular chaperones. Isopeptidases, the enzymes that reverse the actions of CHIP, also play an important role in determining the fate of denatured proteins.
Figures
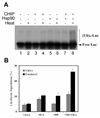





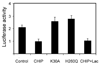

References
-
- Kopito RR. Aggresomes, inclusion bodies and protein aggregation. Trends Cell Biol. 2000;10:524–530. - PubMed
-
- Julien JP. Amyotrophic lateral sclerosis. unfolding the toxicity of the misfolded. Cell. 2001;104:581–591. - PubMed
-
- Ross CA, Pickart CM. The ubiquitin-proteasome pathway in Parkinson's disease and other neurodegenerative diseases. Trends Cell Biol. 2004;14:703–711. - PubMed
-
- Schubert U, Anton LC, Gibbs J, Norbury CC, Yewdell JW, Bennink JR. Rapid degradation of a large fraction of newly synthesized proteins by proteasomes. Nature. 2000;404:770–774. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

