A 9-year-old male with a duplication of chromosome 3p25.3p26.2: clinical report and gene expression analysis
- PMID: 16470700
- PMCID: PMC2568077
- DOI: 10.1002/ajmg.a.31132
A 9-year-old male with a duplication of chromosome 3p25.3p26.2: clinical report and gene expression analysis
Abstract
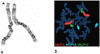
We describe a 9-year-old male referred for genetic evaluation for Prader-Willi syndrome (PWS). PWS is the most common genetically defined cause of life-threatening obesity and results from a functional loss of paternally expressed genes from the chromosome 15q11-q13 region. The patient presented with pervasive developmental disorder, delayed speech, and rapid onset of obesity at age 4 years, all features similar to PWS. However, chromosome 15q11-q13 methylation testing and fragile X studies were normal. GTG-banding and fluorescence in situ hybridization (FISH) with whole chromosome 3 paint probe (WCP3) and a chromosome 3p subtelomeric probe suggested a duplication of 3p25.3p26.2, a finding supported by comparative genomic hybridization (CGH). This region of chromosome 3p contains genes which contribute to obesity and behavioral problems, most notably, ghrelin (GHRL), an oxytocin receptor (OXTR), solute carrier family six members (gamma-aminobutyric acid (GABA) neurotransmitter transporters, SLC6A1 and SLC6A11), and peroxisome proliferator-activated receptor gamma (PPARG). To characterize these obesity and behavior related genes in our subject, we performed quantitative RT-PCR and compared expression levels with similarly aged male subjects (four non-obese males, four obese males, and four PWS males-two with 15q11-q13 deletions and two with maternal disomy 15). Our studies suggest increased expression of several genes in the 3p duplication region, including GHRL and PPARG, which may contribute to the phenotypic features in our 3p duplication subject.
2006 Wiley-Liss, Inc.
Figures




References
-
- Brannan CI, Bartolomei MS. Mechanisms of genomic imprinting. Curr Opin Genet Dev. 1999;9:164–170. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

