Role of cytokinin and auxin in shaping root architecture: regulating vascular differentiation, lateral root initiation, root apical dominance and root gravitropism
- PMID: 16473866
- PMCID: PMC2803412
- DOI: 10.1093/aob/mcl027
Role of cytokinin and auxin in shaping root architecture: regulating vascular differentiation, lateral root initiation, root apical dominance and root gravitropism
Abstract
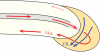
Background and aims: Development and architecture of plant roots are regulated by phytohormones. Cytokinin (CK), synthesized in the root cap, promotes cytokinesis, vascular cambium sensitivity, vascular differentiation and root apical dominance. Auxin (indole-3-acetic acid, IAA), produced in young shoot organs, promotes root development and induces vascular differentiation. Both IAA and CK regulate root gravitropism. The aims of this study were to analyse the hormonal mechanisms that induce the root's primary vascular system, explain how differentiating-protoxylem vessels promote lateral root initiation, propose the concept of CK-dependent root apical dominance, and visualize the CK and IAA regulation of root gravitropiosm.
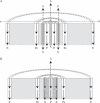
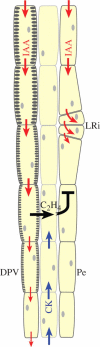
Key issues: The hormonal analysis and proposed mechanisms yield new insights and extend previous concepts: how the radial pattern of the root protoxylem vs. protophloem strands is induced by alternating polar streams of high IAA vs. low IAA concentrations, respectively; how differentiating-protoxylem vessel elements stimulate lateral root initiation by auxin-ethylene-auxin signalling; and how root apical dominance is regulated by the root-cap-synthesized CK, which gives priority to the primary root in competition with its own lateral roots.
Conclusions: CK and IAA are key hormones that regulate root development, its vascular differentiation and root gravitropism; these two hormones, together with ethylene, regulate lateral root initiation.
Figures

References
-
- Aloni R. 1980. Role of auxin and sucrose in the differentiation of sieve and tracheary elements in plant tissue cultures. Planta 150: 255–263. - PubMed
-
- Aloni R. 1987. Differentiation of vascular tissues. Annual Review of Plant Physiology 38: 179–204.
-
- Aloni R. 1991. Wood formation in deciduous hardwood trees. In: Raghavendra AS, ed. Physiology of trees. New York: Wiley, 175–197.
-
- Aloni R. 1995. The induction of vascular tissues by auxin and cytokinin. In: Davies PJ, ed. Plant hormones: physiology, biochemistry and molecular biology. Dordrecht: Kluwer, 531–546.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

