Frequency selectivity of synaptic exocytosis in frog saccular hair cells
- PMID: 16473940
- PMCID: PMC1413814
- DOI: 10.1073/pnas.0511005103
Frequency selectivity of synaptic exocytosis in frog saccular hair cells
Abstract
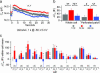
The ability to respond selectively to particular frequency components of sensory inputs is fundamental to signal processing in the ear. The frog (Rana pipiens) sacculus, which is used for social communication and escape behaviors, is an exquisitely sensitive detector of sounds and ground-borne vibrations in the 5- to 200-Hz range, with most afferent axons having best frequencies between 40 and 60 Hz. We monitored the synaptic output of saccular sensory receptors (hair cells) by measuring the increase in membrane capacitance (deltaC(m)) that occurs when synaptic vesicles fuse with the plasmalemma. Strong stepwise depolarization evoked an exocytic burst that lasted 10 ms and corresponded to the predicted capacitance of all docked vesicles at synapses, followed by a 20-ms delay before additional vesicle fusion. Experiments using weak stimuli, within the normal physiological range for these cells, revealed a sensitivity to the temporal pattern of membrane potential changes. Interrupting a weak depolarization with a properly timed hyperpolarization increased deltaC(m). Small sinusoidal voltage oscillations (+/-5 mV centered at -60 mV) evoked a deltaC(m) that corresponded to 95 vesicles per s at each synapse at 50 Hz but only 26 vesicles per s at 5 Hz and 27 vesicles per s at 200 Hz (perforated patch recordings). This frequency selectivity was absent for larger sinusoidal oscillations (+/-10 mV centered at -55 mV) and was largest for hair cells with the smallest sinusoidal-stimuli-evoked Ca2+ currents. We conclude that frog saccular hair cells possess an intrinsic synaptic frequency selectivity that is saturated by strong stimuli.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures
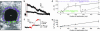




References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

