A macroporous hydrogel for the coculture of neural progenitor and endothelial cells to form functional vascular networks in vivo
- PMID: 16473951
- PMCID: PMC1413771
- DOI: 10.1073/pnas.0506020102
A macroporous hydrogel for the coculture of neural progenitor and endothelial cells to form functional vascular networks in vivo
Abstract
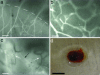
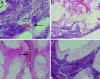
A microvascular network is critical for the survival and function of most tissues. We have investigated the potential of neural progenitor cells to augment the formation and stabilization of microvascular networks in a previously uncharacterized three-dimensional macroporous hydrogel and the ability of this engineered system to develop a functional microcirculation in vivo. The hydrogel is synthesized by cross-linking polyethylene glycol with polylysine around a salt-leached polylactic-co-glycolic acid scaffold that is degraded in a sodium hydroxide solution. An open macroporous network is formed that supports the efficient formation of tubular structures by brain endothelial cells. After subcutaneous implantation of hydrogel cocultures in mice, blood flow in new microvessels was apparent at 2 weeks with perfused networks established on the surface of implants at 6 weeks. Compared to endothelial cells cultured alone, cocultures of endothelial cells and neural progenitor cells had a significantly greater density of tubular structures positive for platelet endothelial cell adhesion molecule-1 at the 6-week time point. In implant cross sections, the presence of red blood cells in vessel lumens confirmed a functional microcirculation. These findings indicate that neural progenitor cells promote the formation of endothelial cell tubes in coculture and the development of a functional microcirculation in vivo. We demonstrate a previously undescribed strategy for creating stable microvascular networks to support engineered tissues of desired parenchymal cell origin.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures



References
-
- Peters M. C., Polverini P. J., Mooney D. J. J. Biomed. Mate. Res. 2002;60:668–678. - PubMed
-
- Smith M. K., Peters M. C., Richardson T. P., Garbern J. C., Mooney D. J. Tissue Eng. 2004;10:63–71. - PubMed
-
- Richardson T. P., Peters M. C., Ennett A. B., Mooney D. J. Nat. Biotechnol. 2001;19:1029–1034. - PubMed
-
- Lee H., Cusick R. A., Browne F., Kim T. H., Ma P. X., Utsunomiya H., Langer R., Vacanti J. P. Transplantation. 2002;73:1589–1593. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

