Role of granulocyte macrophage colony-stimulating factor during gram-negative lung infection with Pseudomonas aeruginosa
- PMID: 16474098
- PMCID: PMC2644237
- DOI: 10.1165/rcmb.2005-0246OC
Role of granulocyte macrophage colony-stimulating factor during gram-negative lung infection with Pseudomonas aeruginosa
Abstract
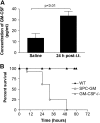
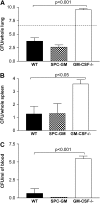
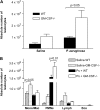
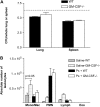
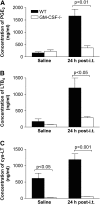
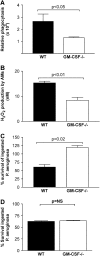
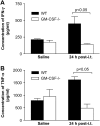
Granulocyte macrophage colony-stimulating factor (GM-CSF) stimulates survival, proliferation, differentiation, and function of myeloid cells. Recently, GM-CSF has been shown to be important for normal pulmonary homeostasis. We report that GM-CSF is induced in lung leukocytes during infection with Gram-negative bacteria. Therefore, we postulated that deficiencies in GM-CSF would increase susceptibility to Gram-negative infection in vivo. After an intratracheal inoculum with Pseudomonas aeruginosa, GM-CSF-/- mice show decreased survival compared with wild-type mice. GM-CSF-/- mice show increased lung, spleen, and blood bacterial CFU. GM-CSF-/- mice are defective in the production of cysteinyl leukotrienes, prostaglandin E2, macrophage inflammatory protein, and keratinocyte-derived chemokine in lung leukocytes postinfection. Despite these defects, inflammatory cell recruitment is not diminished at 6 or 24 h postinfection, and the functional activity of polymorphonuclear leukocytes from the lung and peritoneum against P. aeruginosa is enhanced in GM-CSF-/- mice. In contrast, alveolar macrophage (AM) phagocytosis, killing, and H2O2 production are defective in GM-CSF-/- mice. Although the absence of GM-CSF has profound effects on AMs, peritoneal macrophages seem to have normal bactericidal activities in GM-CSF-/- mice. Defects in AM function may be related to diminished levels of IFN-gamma and TNF-alpha postinfection. Thus, GM-CSF-/- mice are more susceptible to lung infection with P. aeruginosa as a result of impaired AM function.
Figures

References
-
- Holder IA. Pseudomonas immunotherapy: a historical overview. Vaccine 2004;22:831–839. - PubMed
-
- Rolston KV, Bodey GP. Pseudomonas aeruginosa infection in cancer patients. Cancer Invest 1992;10:43–59. - PubMed
-
- Ojielo CI, Cooke K, Mancuso P, Standiford TJ, Olkiewicz KM, Clouthier S, Corrion L, Ballinger MN, Toews GB, Paine R III, et al. Defective phagocytosis and clearance of Pseudomonas aeruginosa in the lung following bone marrow transplantation. J Immunol 2003;171:4416–4424. - PubMed
-
- Vidal F, Mensa J, Martinez JA, Almela M, Marco F, Gatell JM, Richart C, Soriano E, Jimenez de Anta MT. Pseudomonas aeruginosa bacteremia in patients infected with human immunodeficiency virus type 1. Eur J Clin Microbiol Infect Dis 1999;18:473–477. - PubMed
-
- Maschmeyer G, Braveny I. Review of the incidence and prognosis of Pseudomonas aeruginosa infections in cancer patients in the 1990s. Eur J Clin Microbiol Infect Dis 2000;19:915–925. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

