Herpes simplex virus type 1 DNA-packaging protein UL17 is required for efficient binding of UL25 to capsids
- PMID: 16474120
- PMCID: PMC1395399
- DOI: 10.1128/JVI.80.5.2118-2126.2006
Herpes simplex virus type 1 DNA-packaging protein UL17 is required for efficient binding of UL25 to capsids
Abstract
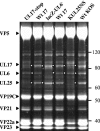
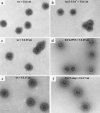
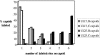
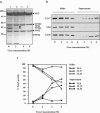
Herpes simplex virus type 1 packages its DNA genome into a precursor capsid, referred to as the procapsid. Of the three capsid-associated DNA-packaging proteins, UL17, UL25, and UL6, only UL17 and UL6 appear to be components of the procapsid, with UL25 being added subsequently. To determine whether the association of UL17 or UL25 with capsids was dependent on the other two packaging proteins, B capsids, which lack viral DNA but retain the cleaved internal scaffold, were purified from nonpermissive cells infected with UL17, UL25, or UL6 null mutants and compared with wild-type (wt) B capsids. In the absence of UL17, the levels of UL25 in the mutant capsids were much lower than those in wt B capsids. These results suggest that UL17 is required for efficient incorporation of UL25 into B capsids. B capsids lacking UL25 contained about twofold-less UL17 than wt capsids, raising the possibilities that UL25 is important for stabilizing UL17 in capsids and that the two proteins interact in the capsid. The distribution of UL17 and UL25 on B capsids was examined using immunogold labeling. Both proteins appeared to bind to multiple sites on the capsid. The properties of the UL17 and UL25 proteins are consistent with the idea that the two proteins are important in stabilizing capsid-DNA structures rather than having a direct role in DNA packaging.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

