RNA secondary structure in the coding region of dengue virus type 2 directs translation start codon selection and is required for viral replication
- PMID: 16474125
- PMCID: PMC1395379
- DOI: 10.1128/JVI.80.5.2170-2182.2006
RNA secondary structure in the coding region of dengue virus type 2 directs translation start codon selection and is required for viral replication
Abstract
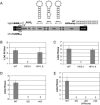
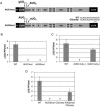
Dengue virus is a positive-strand RNA virus and a member of the genus Flavivirus, which includes West Nile, yellow fever, and tick-borne encephalitis viruses. Flavivirus genomes are translated as a single polyprotein that is subsequently cleaved into 10 proteins, the first of which is the viral capsid (C) protein. Dengue virus type 2 (DENV2) and other mosquito-borne flaviviruses initiate translation of C from a start codon in a suboptimal context and have multiple in-frame AUGs downstream. Here, we show that an RNA hairpin structure in the capsid coding region (cHP) directs translation start site selection in human and mosquito cells. The ability of the cHP to direct initiation from the first start codon is proportional to its thermodynamic stability, is position dependent, and is sequence independent, consistent with a mechanism in which the scanning initiation complex stalls momentarily over the first AUG as it begins to unwind the cHP. The cHP of tick-borne flaviviruses is not maintained in a position to influence start codon selection, which suggests that this coding region cis element may serve another function in the flavivirus life cycle. Here, we demonstrate that the DENV2 cHP and both the first and second AUGs of C are necessary for efficient viral replication in human and mosquito cells. While numerous regulatory elements have been identified in the untranslated regions of RNA viral genomes, we show that the cHP is a coding-region RNA element that directs start codon selection and is required for viral replication.
Figures






References
-
- Alvarez, D. E., A. L. De Lella Ezcurra, S. Fucito, and A. V. Gamarnik. 2005. Role of RNA structures present at the 3′UTR of dengue virus on translation, RNA synthesis, and viral replication. Virology 339:200-212. - PubMed
-
- Bampi, C., S. Jacquenet, D. Lener, D. Decimo, and J. L. Darlix. 2004. The chaperoning and assistance roles of the HIV-1 nucleocapsid protein in proviral DNA synthesis and maintenance. Int. J. Biochem. Cell. Biol. 36:1668-1686. - PubMed
-
- Bell, J. R., R. M. Kinney, D. W. Trent, E. M. Lenches, L. Dalgarno, and J. H. Strauss. 1985. Amino-terminal amino acid sequences of structural proteins of three flaviviruses. Virology 143:224-229. - PubMed
-
- Bulich, R., and J. G. Aaskov. 1992. Nuclear localization of dengue 2 virus core protein detected with monoclonal antibodies. J. Gen. Virol. 73:2999-3003. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

