Transcriptional origin of Kaposi's sarcoma-associated herpesvirus microRNAs
- PMID: 16474131
- PMCID: PMC1395403
- DOI: 10.1128/JVI.80.5.2234-2242.2006
Transcriptional origin of Kaposi's sarcoma-associated herpesvirus microRNAs
Abstract
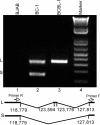
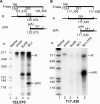
Kaposi's sarcoma-associated herpesvirus (KSHV) encodes 11 distinct microRNAs, all of which are found clustered within the major latency-associated region of the KSHV genome in the same transcriptional orientation. Because the KSHV microRNAs are all expressed in latently infected cells and are largely unaffected by induction of lytic replication, it appeared probable that they would be processed out of KSHV transcripts that are derived from a latent promoter(s) present in this region. Here, we define three latent transcripts, derived from two distinct KSHV latent promoters, that function as both KSHV primary microRNA precursors and as kaposin pre-mRNAs. These activities require the readthrough of a leaky viral polyadenylation signal located at nucleotide 122070 in the KSHV genome. In contrast, recognition of this polyadenylation signal gives rise to previously identified mRNAs that encode the KSHV open reading frames (ORFs) 71, 72 and 73 proteins as well as a novel unspliced KSHV mRNA that encodes only ORF72 and ORF71. Thus, transcripts initiating at the two latent promoters present in the KSHV latency-associated region can undergo two entirely distinct fates, i.e., processing to give a kaposin mRNA and viral microRNAs on the one hand or expression as KSHV ORF71, ORF72, or ORF73 mRNAs on the other, depending on whether the viral polyadenylation site located at position 122070 is ignored or recognized, respectively.
Figures





References
-
- Bartel, D. P. 2004. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116:281-297. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

