A single intranasal inoculation with a paramyxovirus-vectored vaccine protects guinea pigs against a lethal-dose Ebola virus challenge
- PMID: 16474134
- PMCID: PMC1395378
- DOI: 10.1128/JVI.80.5.2267-2279.2006
A single intranasal inoculation with a paramyxovirus-vectored vaccine protects guinea pigs against a lethal-dose Ebola virus challenge
Abstract
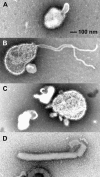
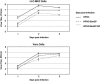
To determine whether intranasal inoculation with a paramyxovirus-vectored vaccine can induce protective immunity against Ebola virus (EV), recombinant human parainfluenza virus type 3 (HPIV3) was modified to express either the EV structural glycoprotein (GP) by itself (HPIV3/EboGP) or together with the EV nucleoprotein (NP) (HPIV3/EboGP-NP). Expression of EV GP by these recombinant viruses resulted in its efficient incorporation into virus particles and increased cytopathic effect in Vero cells. HPIV3/EboGP was 100-fold more efficiently neutralized by antibodies to EV than by antibodies to HPIV3. Guinea pigs infected with a single intranasal inoculation of 10(5.3) PFU of HPIV3/EboGP or HPIV3/EboGP-NP showed no apparent signs of disease yet developed a strong humoral response specific to the EV proteins. When these animals were challenged with an intraperitoneal injection of 10(3) PFU of EV, there were no outward signs of disease, no viremia or detectable EV antigen in the blood, and no evidence of infection in the spleen, liver, and lungs. In contrast, all of the control animals died or developed severe EV disease following challenge. The highly effective immunity achieved with a single vaccine dose suggests that intranasal immunization with live vectored vaccines based on recombinant respiratory viruses may be an advantageous approach to inducing protective responses against severe systemic infections, such as those caused by hemorrhagic fever agents.
Figures







References
-
- Baize, S., E. M. Leroy, M. C. Georges-Courbot, M. Capron, J. Lansoud-Soukate, P. Debre, S. P. Fisher-Hoch, J. B. McCormick, and A. J. Georges. 1999. Defective humoral responses and extensive intravascular apoptosis are associated with fatal outcome in Ebola virus-infected patients. Nat. Med. 5:423-426. - PubMed
-
- Baize, S., E. M. Leroy, E. Mavoungou, and S. P. Fisher-Hoch. 2000. Apoptosis in fatal Ebola infection. Does the virus toll the bell for immune system? Apoptosis 5:5-7. - PubMed
-
- Bosio, C. M., M. J. Aman, C. Grogan, R. Hogan, G. Ruthel, D. Negley, M. Mohamadzadeh, S. Bavari, and A. Schmaljohn. 2003. Ebola and Marburg viruses replicate in monocyte-derived dendritic cells without inducing the production of cytokines and full maturation. J. Infect. Dis. 188:1630-1638. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

