Ataxia-telangiectasia-mutated (ATM) protein can enhance human immunodeficiency virus type 1 replication by stimulating Rev function
- PMID: 16474151
- PMCID: PMC1395391
- DOI: 10.1128/JVI.80.5.2445-2452.2006
Ataxia-telangiectasia-mutated (ATM) protein can enhance human immunodeficiency virus type 1 replication by stimulating Rev function
Abstract
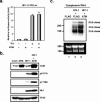
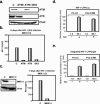
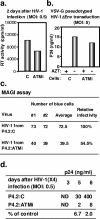
The ataxia-telangiectasia-mutated (ATM) kinase plays a central role in responses to various forms of DNA damage and has been suggested to facilitate human immunodeficiency virus type 1 (HIV-1) integration. Here, we describe a series of experiences that indicate that ATM can enhance HIV-1 replication by stimulating the action of the Rev viral posttranscriptional regulator. The Rev-dependent stimulation of viral late gene expression was observed with ATM-overexpressing cells, a result confirmed with a Rev-dependent reporter construct. Both parameters were also enhanced upon treatment of HeLa cells with caffeine, a xanthine that, in this cellular context, stimulates ATM activity. As well, decreased levels of virions with reduced infectivity were released by ATM knockdown cells. Notably, ATM overexpression did not stimulate the HIV-1 late gene expression within the context of Rev-independent constructs or the Rex-dependent production of capsid from human T-cell leukemia virus type 1 proviral constructs. Altogether, these results indicate that ATM can positively influence HIV-1 Rev function.
Figures




References
-
- Aiken, C., L. Krause, Y.-L. Chen, and D. Trono. 1996. Mutational analysis of HIV-1 Nef: identification of two mutants that are temperature-sensitive for CD4 downregulation. Virology 217:293-300. - PubMed
-
- Ariumi, Y., A. Kaida, M. Hatanaka, and K. Shimotohno. 2001. Functional cross-talk of HIV-1 Tat with p53 through its C-terminal domain. Biochem. Biophys. Res. Commun. 287:556-561. - PubMed
-
- Bres, V., R. E. Kiernan, L. K. Linares, C. Chable-Bessia, O. Plechakova, C. Treand, S. Emiliani, J. M. Peloponese, K. T. Jeang, O. Coux, M. Scheffner, and M. Benkirane. 2003. A non-proteolytic role for ubiquitin in Tat-mediated transactivation of the HIV-1 promoter. Nat. Cell Biol. 5:754-761. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

