Preferential infection of mature dendritic cells by mouse hepatitis virus strain JHM
- PMID: 16474157
- PMCID: PMC1395395
- DOI: 10.1128/JVI.80.5.2506-2514.2006
Preferential infection of mature dendritic cells by mouse hepatitis virus strain JHM
Abstract
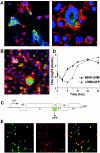
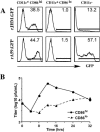
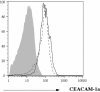
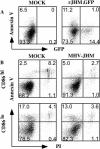
Mouse hepatitis virus strain JHM (MHV-JHM) causes acute encephalitis and acute and chronic demyelinating diseases in mice. Dendritic cells (DCs) are key cells in the initiation of innate and adaptive immune responses, and infection of these cells could potentially contribute to a dysregulated immune response; consistent with this, recent results suggest that DCs are readily infected by another strain of mouse hepatitis virus, the A59 strain (MHV-A59). Herein, we show that the JHM strain also productively infected DCs. Moreover, mature DCs were at least 10 times more susceptible than immature DCs to infection with MHV-JHM. DC function was impaired after MHV-JHM infection, resulting in decreased stimulation of CD8 T cells in vitro. Preferential infection of mature DCs was not due to differential expression of the MHV-JHM receptor CEACAM-1a on mature or immature cells or to differences in apoptosis. Although we could not detect infected DCs in vivo, both CD8(+) and CD11b(+) splenic DCs were susceptible to infection with MHV-JHM directly ex vivo. This preferential infection of mature DCs may inhibit the development of an efficient immune response to the virus.
Figures


References
-
- Andrews, D. M., C. E. Andoniou, F. Granucci, P. Ricciardi-Castagnoli, and M. A. Degli-Esposti. 2001. Infection of dendritic cells by murine cytomegalovirus induces functional paralysis. Nat. Immunol. 2:1077-1084. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

