Mechanism of hERG K+ channel blockade by the fluoroquinolone antibiotic moxifloxacin
- PMID: 16474415
- PMCID: PMC1760709
- DOI: 10.1038/sj.bjp.0706678
Mechanism of hERG K+ channel blockade by the fluoroquinolone antibiotic moxifloxacin
Abstract
The fluoroquinolone antibiotic moxifloxacin has been associated with the acquired long QT syndrome and is used as a positive control in the evaluation of the QT-interval prolonging potential of new drugs. In common with other QT-prolonging agents, moxifloxacin is known to inhibit the hERG potassium K+ channel, but at present there is little mechanistic information available on this action. This study was conducted in order to characterise the inhibition of hERG current (I(hERG)) by moxifloxacin, and to determine the role in drug binding of the S6 aromatic amino-acid residues Tyr652 and Phe656. hERG currents were studied using whole-cell patch clamp (at room temperature and at 35-37 degrees C) in an HEK293 cell line stably expressing hERG channels. Moxifloxacin reversibly inhibited currents in a dose-dependent manner. We investigated the effects of different voltage commands to elicit hERG currents on moxifloxacin potency. Using a 'step-ramp' protocol, the IC50 was 65 microM at room temperature and 29 microM at 35 degrees C. When a ventricular action potential waveform was used to elicit currents, the IC50 was 114 microM. Block of hERG by moxifloxacin was found to be voltage-dependent, occurred rapidly and was independent of stimulation frequency. Mutagenesis of the S6 helix residue Phe656 to Ala failed to eliminate or reduce the moxifloxacin-mediated block whereas mutation of Tyr652 to Ala reduced moxifloxacin block by approximately 66%. Our data demonstrate that moxifloxacin blocks the hERG channel with a preference for the activated channel state. The Tyr652 but not Phe656 S6 residue is involved in moxifloxacin block of hERG, concordant with an interaction in the channel inner cavity.
Figures

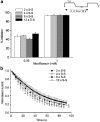
 where A1 and A2 are 0 and 100% inhibition, respectively, C is the IC50 concentration and nH is the Hill slope. The IC50 values yielded under these two experimental conditions were statistically different from one another (P<0.001). The inset shows the concentration–response curve at 22°C for levofloxacin generated using the step-ramp voltage protocol. Data are from six cells and a Hill equation was fitted as for moxifloxacin.
where A1 and A2 are 0 and 100% inhibition, respectively, C is the IC50 concentration and nH is the Hill slope. The IC50 values yielded under these two experimental conditions were statistically different from one another (P<0.001). The inset shows the concentration–response curve at 22°C for levofloxacin generated using the step-ramp voltage protocol. Data are from six cells and a Hill equation was fitted as for moxifloxacin.
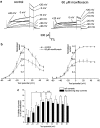
 where A1 and A2 are 0 and 1 respectively, V1/2 is the half-activation voltage and k is the slope factor. (c) Mean % inhibition of both tail currents (open bars) and depolarising step currents (solid bars) were calculated at each test potential and are summarised here.
where A1 and A2 are 0 and 1 respectively, V1/2 is the half-activation voltage and k is the slope factor. (c) Mean % inhibition of both tail currents (open bars) and depolarising step currents (solid bars) were calculated at each test potential and are summarised here.


References
-
- AMANKWA K., KRISHNAN S.C., TISDALE J.E. Torsades de pointes associated with fluoroquinolones: importance of concomitant risk factors. Clin.l Pharmacol. Ther. 2004;75:242–247. - PubMed
-
- BARRIERE S., GENTER F., SPENCER E., KITT M., HOELSCHER D., MORGANROTH J. Effects of a new antibacterial, telavancin, on cardiac repolarization (QTc interval duration) in healthy subjects. J. Clin. Pharmacol. 2004;44:689–695. - PubMed
-
- BARRY P.H. JPCalc, a software package for calculating liquid junction potential corrections in patch-clamp, intracellular, epithelial and bilayer measurements and for correcting junction potential measurements. J. Neurosci. Methods. 1994;51:107–116. - PubMed
-
- BISCHOFF U., SCHMIDT C., NETZER R., PONGS O. Effects of fluoroquinolones on HERG currents. Eur. J. Pharmacol. 2000;406:341–343. - PubMed
-
- BOSCH R.F., GASPO R., BUSCH A.E., LANG H.J., LI G.-R., NATTEL S. Effects of the chromanol 293B, a selective blocker of the slow, component of the delayed rectifier K+ current, on repolarization in human and guinea pig ventricular myocytes. Cardiovasc. Res. 1998;38:441–450. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

