Complex regulation of human androgen receptor expression by Wnt signaling in prostate cancer cells
- PMID: 16474850
- PMCID: PMC2630384
- DOI: 10.1038/sj.onc.1209366
Complex regulation of human androgen receptor expression by Wnt signaling in prostate cancer cells
Erratum in
- Oncogene. 2006 Jul 13;25(30):4256
Abstract
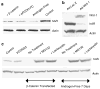
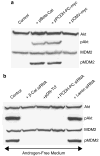
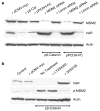
beta-Catenin, a component of the Wnt signaling pathway, is a coactivator of human androgen receptor (hAR) transcriptional activity. Here, we show that Wnt signaling also influences androgen-mediated signaling through its ability to regulate hAR mRNA and protein in prostate cancer (PCa) cells. Three functional LEF-1/TCF binding sites lie within the promoter of the hAR gene as shown by CHIP assays that captured beta-catenin-bound chromatin from Wnt-activated LNCaP cells. Chimeric reporter vectors that use the hAR gene promoter to drive luciferase expression confirmed that these LEF-1/TCF binding elements are able to confer robust upregulation of luciferase expression when stimulated by Wnt-1 or by transfection with beta-catenin and that dominant-negative TCF or mutations within the dominant TCF-binding element abrogated the response. Semi-quantitative and real time RT-PCR assays confirmed that Wnt activation upregulates hAR mRNA in PCa cells. In contrast, hAR protein expression was strongly suppressed by Wnt activation. The reduction of hAR protein is consistent with evidence that Wnt signaling increased phosphorylation of Akt and its downstream target, MDM2 that promotes degradation of hAR protein through a proteasomal pathway. These data indicate that the hAR gene is a direct target of LEF-1/TCF transcriptional regulation in PCa cells but also show that the expression of the hAR protein is suppressed by a degradation pathway regulated by cross-talk of Wnt to Akt that is likely mediated by Wnt-directed degradation of the B regulatory subunit of protein phosphatase, PP2A.
Figures



References
-
- Ashcroft M, Ludwig RL, Woods DB, Copeland TD, Weber HO, MacRae EJ, et al. Oncogene. 2002;21:1955–1962. - PubMed
-
- Barker N, Clevers H. Bioessays. 2000;22:961–965. - PubMed
-
- Chang C, Saltzman A, Yeh S, Young W, Keller E, Lee H-J, et al. Crit Rev Eukaryot Gene Exp. 1995;5:97–125. - PubMed
-
- Chen CD, Welsbie DS, Tran C, Baek SH, Chen R, Vessella R, et al. Nature Med. 2004a;10:33–39. - PubMed
-
- Chen G, Shukeir N, Potti A, Sircare K, Aprikian A, Goltzman D, et al. Cancer. 2004b;10:1345–1356. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

