Modelling insecticide-binding sites in the voltage-gated sodium channel
- PMID: 16475981
- PMCID: PMC1462714
- DOI: 10.1042/BJ20051925
Modelling insecticide-binding sites in the voltage-gated sodium channel
Abstract
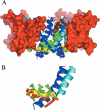
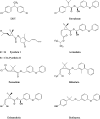
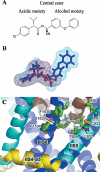
A homology model of the housefly voltage-gated sodium channel was developed to predict the location of binding sites for the insecticides fenvalerate, a synthetic pyrethroid, and DDT an early generation organochlorine. The model successfully addresses the state-dependent affinity of pyrethroid insecticides, their mechanism of action and the role of mutations in the channel that are known to confer insecticide resistance. The sodium channel was modelled in an open conformation with the insecticide-binding site located in a hydrophobic cavity delimited by the domain II S4-S5 linker and the IIS5 and IIIS6 helices. The binding cavity is predicted to be accessible to the lipid bilayer and therefore to lipid-soluble insecticides. The binding of insecticides and the consequent formation of binding contacts across different channel elements could stabilize the channel when in an open state, which is consistent with the prolonged sodium tail currents induced by pyrethroids and DDT. In the closed state, the predicted alternative positioning of the domain II S4-S5 linker would result in disruption of pyrethroid-binding contacts, consistent with the observation that pyrethroids have their highest affinity for the open channel. The model also predicts a key role for the IIS5 and IIIS6 helices in insecticide binding. Some of the residues on the helices that form the putative binding contacts are not conserved between arthropod and non-arthropod species, which is consistent with their contribution to insecticide species selectivity. Additional binding contacts on the II S4-S5 linker can explain the higher potency of pyrethroid insecticides compared with DDT.
Figures




References
-
- Wang S. Y., Wang G. K. Voltage-gated sodium channels as primary targets of diverse lipid-soluble neurotoxins. Cell. Signalling. 2003;15:151–159. - PubMed
-
- Catterall W. A. Neurotoxins that act on voltage-sensitive sodium-channels in excitable-membranes. Annu. Rev. Pharmacol. Toxicol. 1980;20:15–43. - PubMed
-
- Trainer V. L., McPhee J. C., Boutelet-Bochan H., Baker C., Scheuer T., Babin D., Demoute J. P., Guedin D., Catterall W. A. High affinity binding of pyrethroids to the α-subunit of brain sodium channels. Mol. Pharmacol. 1997;51:651–657. - PubMed
-
- Leibowitz M. D., Schwarz J. R., Holan G., Hille B. Distinct modifications of sodium channels by alkaloids and insecticides. Biophys. J. 1986;49:A43.
-
- Salgado V. L., Narahashi T. Immobilization of sodium-channel gating charge in crayfish giant-axons by the insecticide fenvalerate. Mol. Pharmacol. 1993;43:626–634. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

