Luminal bacterial antigen-specific CD4+ T-cell responses in HLA-B27 transgenic rats with chronic colitis are mediated by both major histocompatibility class II and HLA-B27 molecules
- PMID: 16476051
- PMCID: PMC1782237
- DOI: 10.1111/j.1365-2567.2005.02303.x
Luminal bacterial antigen-specific CD4+ T-cell responses in HLA-B27 transgenic rats with chronic colitis are mediated by both major histocompatibility class II and HLA-B27 molecules
Abstract
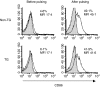
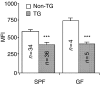
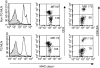
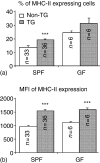
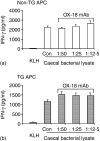
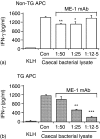
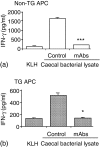
Rats transgenic (TG) for the human major histocompatibility complex (MHC) class I HLA-B27 and beta2-microglobulin genes develop chronic colitis under specific pathogen-free (SPF) but not sterile (germ-free, GF) conditions. We investigated the role of antigen-presenting molecules involved in generating immune responses by CD4+ mesenteric lymph node (MLN) cells from colitic HLA-B27 TG rats to commensal enteric micro-organisms. All TG MLN cells expressed HLA-B27. A higher level of MHC class II was expressed on cells from TG rats, both SPF and GF, compared to non-TG littermates. In contrast, rat MHC class I expression was lower on TG than non-TG cells. Both TG and non-TG antigen presenting cells (APC) pulsed with caecal bacterial antigens induced a marked interferon-gamma (IFN-gamma) response in TG CD4+ T lymphocytes but failed to stimulate non-TG cells. Blocking MHC class II on both TG and non-TG APC dramatically inhibited their ability to induce TG CD4+ T cells to produce IFN-gamma. Blocking HLA-B27 on TG APC similarly inhibited IFN-gamma responses. When the antibodies against MHC class II and HLA-B27 were combined, no APC-dependent IFN-gamma response was detected. These data implicate both native rat MHC class II and TG HLA-B27 in CD4+ MLN T-cell IFN-gamma responses to commensal enteric microflora in this colitis model.
Figures


Similar articles
-
Dysregulated luminal bacterial antigen-specific T-cell responses and antigen-presenting cell function in HLA-B27 transgenic rats with chronic colitis.Immunology. 2005 Sep;116(1):112-21. doi: 10.1111/j.1365-2567.2005.02206.x. Immunology. 2005. PMID: 16108823 Free PMC article.
-
Reduced ratio of protective versus proinflammatory cytokine responses to commensal bacteria in HLA-B27 transgenic rats.Clin Exp Immunol. 2004 Apr;136(1):30-9. doi: 10.1111/j.1365-2249.2004.02410.x. Clin Exp Immunol. 2004. PMID: 15030511 Free PMC article.
-
CD4(+) T lymphocytes mediate colitis in HLA-B27 transgenic rats monoassociated with nonpathogenic Bacteroides vulgatus.Inflamm Bowel Dis. 2007 Mar;13(3):317-24. doi: 10.1002/ibd.20040. Inflamm Bowel Dis. 2007. PMID: 17206701
-
The recognition of abnormal forms of HLA-B27 by CD4+ T cells.Curr Mol Med. 2004 Feb;4(1):51-8. doi: 10.2174/1566524043479257. Curr Mol Med. 2004. PMID: 15011959 Review.
-
Unraveling the mystery of HLA-B27 association with human spondyloarthropathies using transgenic and knock out mice.Semin Immunol. 1998 Feb;10(1):15-23. doi: 10.1006/smim.1997.0101. Semin Immunol. 1998. PMID: 9529652 Review.
Cited by
-
Lessons on SpA pathogenesis from animal models.Semin Immunopathol. 2021 Apr;43(2):207-219. doi: 10.1007/s00281-020-00832-x. Epub 2021 Jan 15. Semin Immunopathol. 2021. PMID: 33449154 Review.
-
Adoptive transfer of nontransgenic mesenteric lymph node cells induces colitis in athymic HLA-B27 transgenic nude rats.Clin Exp Immunol. 2006 Mar;143(3):474-83. doi: 10.1111/j.1365-2249.2006.03013.x. Clin Exp Immunol. 2006. PMID: 16487247 Free PMC article.
-
Spondyloarthritides: Theories and beyond.Front Pediatr. 2022 Dec 23;10:1074239. doi: 10.3389/fped.2022.1074239. eCollection 2022. Front Pediatr. 2022. PMID: 36619518 Free PMC article. Review.
References
-
- Sartor RB. Microbial influences in inflammatory bowel disease: role in pathogenesis and clinical implications. In: Sartor RB, Sandborn WJ, editors. Kirsner's Inflammatory Bowel Diseases. Philadelphia: Elsevier; 2004. pp. 138–62.
-
- Sartor RB. Animal models of intestinal inflammation. In: Sartor RB, Sandborn WJ, editors. Kirsner's Inflammatory Bowel Diseases. Philadelphia: Elsevier; 2004. pp. 120–37.
-
- Davidson NJ, Hudak SA, Lesley RE, Menon S, Leach MW, Rennick DM. IL-12, but not IFN-gamma, plays a major role in sustaining the chronic phase of colitis in IL-10-deficient mice. J Immunol. 1998;161:3143–9. - PubMed
-
- Powrie F, Leach MW, Mauze S, Menon S, Caddle LB, Coffman RL. Inhibition of Th1 responses prevents inflammatory bowel disease in scid mice reconstituted with CD45RBhi CD4+ T cells. Immunity. 1994;1:553–62. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

