Penetratin tandemly linked to a CTL peptide induces anti-tumour T-cell responses via a cross-presentation pathway
- PMID: 16476052
- PMCID: PMC1782229
- DOI: 10.1111/j.1365-2567.2005.02304.x
Penetratin tandemly linked to a CTL peptide induces anti-tumour T-cell responses via a cross-presentation pathway
Abstract
Recently there has been increasing evidence to suggest that membrane translocating peptides enter cells by a receptor-dependent pathway. There have been some studies on the mechanism of major histocompatibility complex (MHC) class I presentation of membrane translocating peptides incorporating cytotoxic T lymphocyte epitopes. However, these have been on different cell lines and only a limited number of inhibitors of the antigen presentation pathway were used. Herein, we demonstrate a comprehensive study utilizing a full spectrum of inhibitors to various pathways of MHC class I to elucidate the mechanism of the membrane translocating peptide, penetratin from Antennapedia (Int). It is clear that Int, RQIKIWFQNRRMKWKK when tandemly linked to a cytotoxic T lymphocyte peptide of ovalbumin, SIINFEKL (IntSIIN) is endocytosed via phagocytosis or macropinocytosis by dendritic cells in an ATP-dependent manner and is processed by a proteasome- and tapasin-independent pathway for presentation and loading to MHC class I molecules. In addition, the majority of antigen is taken up by negatively charged receptors. IntSIIN activates T cells in vitro and in vivo and protects mice against challenge with an ovalbumin-expressing tumour.
Figures
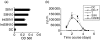

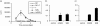
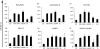
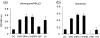
References
-
- Trombetta ES, Mellman I. Cell biology of antigen processing in vitro and in vivo. Annu Rev Immunol. 2005;23:975–1028. - PubMed
-
- Apostolopoulos V, Barnes N, Pietersz GA, McKenzie IF. Ex vivo targeting of the macrophage mannose receptor generates anti-tumor CTL responses. Vaccine. 2000;18:3174–84. - PubMed
-
- Apostolopoulos V, Lofthouse SA, Popovski V, Chelvanayagam G, Sandrin MS, McKenzie IF. Peptide mimics of a tumor antigen induce functional cytotoxic T cells. Nat Biotechnol. 1998;16:276–80. - PubMed
-
- Apostolopoulos V, McKenzie IF. Cellular mucins: targets for immunotherapy. Crit Rev Immunol. 1994;14:293–309. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

