Canine parvovirus-like particles, a novel nanomaterial for tumor targeting
- PMID: 16476163
- PMCID: PMC1386698
- DOI: 10.1186/1477-3155-4-2
Canine parvovirus-like particles, a novel nanomaterial for tumor targeting
Abstract
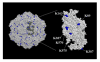
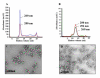
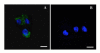
Specific targeting of tumor cells is an important goal for the design of nanotherapeutics for the treatment of cancer. Recently, viruses have been explored as nano-containers for specific targeting applications, however these systems typically require modification of the virus surface using chemical or genetic means to achieve tumor-specific delivery. Interestingly, there exists a subset of viruses with natural affinity for receptors on tumor cells that could be exploited for nanotechnology applications. For example, the canine parvovirus (CPV) utilizes transferrin receptors (TfRs) for binding and cell entry into canine as well as human cells. TfRs are over-expressed by a variety of tumor cells and are widely being investigated for tumor-targeted drug delivery. We explored whether the natural tropism of CPV to TfRs could be harnessed for targeting tumor cells. Towards this goal, CPV virus-like particles (VLPs) produced by expression of the CPV-VP2 capsid protein in a baculovirus expression system were examined for attachment of small molecules and delivery to tumor cells. Structural modeling suggested that six lysines per VP2 subunit are presumably addressable for bioconjugation on the CPV capsid exterior. Between 45 and 100 of the possible 360 lysines/particle could be routinely derivatized with dye molecules depending on the conjugation conditions. Dye conjugation also demonstrated that the CPV-VLPs could withstand conditions for chemical modification on lysines. Attachment of fluorescent dyes neither impaired binding to the TfRs nor affected internalization of the 26 nm-sized VLPs into several human tumor cell lines. CPV-VLPs therefore exhibit highly favorable characteristics for development as a novel nanomaterial for tumor targeting.
Figures
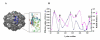



References
-
- Fennelly D. Dose intensity in advanced ovarian cancer: have we answered the question? Clin Cancer Res. 1995;1:575–582. - PubMed
-
- Myers CE, Chabner BA. Anthracyclins. In: Chabner, B. A., Collins, J. M., editor. Cancer chemotherapy-principles and practice. Philadelphia, Lippincott; 1990. pp. 256–381.
-
- Feng SS, Chien S. Chemotherapeutic engineering: application and further development of chemical engineering principles for chemotherapy of cancer and other diseases. Chemical Engineering Science. 2003;58:4087–4114. doi: 10.1016/S0009-2509(03)00234-3. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

