Isoprenoids determine Th1/Th2 fate in pathogenic T cells, providing a mechanism of modulation of autoimmunity by atorvastatin
- PMID: 16476765
- PMCID: PMC2118212
- DOI: 10.1084/jem.20051129
Isoprenoids determine Th1/Th2 fate in pathogenic T cells, providing a mechanism of modulation of autoimmunity by atorvastatin
Abstract
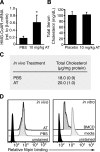
3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase is a critical enzyme in the mevalonate pathway that regulates the biosynthesis of cholesterol as well as isoprenoids that mediate the membrane association of certain GTPases. Blockade of this enzyme by atorvastatin (AT) inhibits the destructive proinflammatory T helper cell (Th)1 response during experimental autoimmune encephalomyelitis and may be beneficial in the treatment of multiple sclerosis and other Th1-mediated autoimmune diseases. Here we present evidence linking specific isoprenoid intermediates of the mevalonate pathway to signaling pathways that regulate T cell autoimmunity. We demonstrate that the isoprenoid geranylgeranyl-pyrophosphate (GGPP) mediates proliferation, whereas both GGPP and its precursor, farnesyl-PP, regulate the Th1 differentiation of myelin-reactive T cells. Depletion of these isoprenoid intermediates in vivo via oral AT administration hindered these T cell responses by decreasing geranylgeranylated RhoA and farnesylated Ras at the plasma membrane. This was associated with reduced extracellular signal-regulated kinase (ERK) and p38 phosphorylation and DNA binding of their cotarget c-fos in response to T cell receptor activation. Inhibition of ERK and p38 mimicked the effects of AT and induced a Th2 cytokine shift. Thus, by connecting isoprenoid availability to regulation of Th1/Th2 fate, we have elucidated a mechanism by which AT may suppress Th1-mediated central nervous system autoimmune disease.
Figures






References
-
- Vollmer, T., L. Key, V. Durkalski, W. Tyor, and J. Corboy. 2004. Oral simvastatin treatment in relapsing-remitting multiple sclerosis. Lancet. 363:1607–1608. - PubMed
-
- McCarey, D.W., I.B. McInnes, R. Madhok, R. Hampson, O. Scherbakov, I. Ford, H.A. Capell, and N. Sattar. 2004. Trial of atorvastatin in Rheumatoid Arthritis (TARA): double-blind, randomised placebo-controlled trial. Lancet. 363:2015–2021. - PubMed
-
- Neuhaus, O., O. Stuve, S.S. Zamvil, and H.-P. Hartung. 2004. Are statins a treatment option for multiple sclerosis. Lancet Neurol. 3:369–371. - PubMed
-
- Stanislaus, R., K. Pahan, A.K. Singh, and I. Singh. 1999. Amelioration of experimental allergic encephalomyelitis in Lewis rats by lovastatin. Neurosci. Lett. 269:71–74. - PubMed
-
- Youssef, S., O. Stuve, J.C. Patarroyo, P.J. Ruiz, J.L. Radosevich, E.M. Hur, M. Bravo, D. Mitchell, R.A. Sobel, L. Steinman, and S.S. Zamvil. 2002. The HMG-CoA reductase inhibitor atorvastatin, promotes Th2 bias and reverses paralysis in central nervous system autoimmune disease. Nature. 420:78–84. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

