A repeated IMP-binding motif controls oskar mRNA translation and anchoring independently of Drosophila melanogaster IMP
- PMID: 16476777
- PMCID: PMC2063677
- DOI: 10.1083/jcb.200510044
A repeated IMP-binding motif controls oskar mRNA translation and anchoring independently of Drosophila melanogaster IMP
Abstract
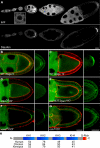
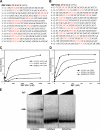
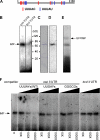
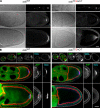
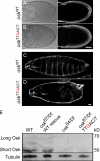
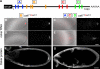
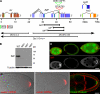
Zip code-binding protein 1 (ZBP-1) and its Xenopus laevis homologue, Vg1 RNA and endoplasmic reticulum-associated protein (VERA)/Vg1 RNA-binding protein (RBP), bind repeated motifs in the 3' untranslated regions (UTRs) of localized mRNAs. Although these motifs are required for RNA localization, the necessity of ZBP-1/VERA remains unresolved. We address the role of ZBP-1/VERA through analysis of the Drosophila melanogaster homologue insulin growth factor II mRNA-binding protein (IMP). Using systematic evolution of ligands by exponential enrichment, we identified the IMP-binding element (IBE) UUUAY, a motif that occurs 13 times in the oskar 3'UTR. IMP colocalizes with oskar mRNA at the oocyte posterior, and this depends on the IBEs. Furthermore, mutation of all, or subsets of, the IBEs prevents oskar mRNA translation and anchoring at the posterior. However, oocytes lacking IMP localize and translate oskar mRNA normally, illustrating that one cannot necessarily infer the function of an RBP from mutations in its binding sites. Thus, the translational activation of oskar mRNA must depend on the binding of another factor to the IBEs, and IMP may serve a different purpose, such as masking IBEs in RNAs where they occur by chance. Our findings establish a parallel requirement for IBEs in the regulation of localized maternal mRNAs in D. melanogaster and X. laevis.
Figures
References
-
- Bergsten, S.E., and E.R. Gavis. 1999. Role for mRNA localization in translational activation but not spatial restriction of nanos RNA. Development. 126:659–669. - PubMed
-
- Brand, A.H., and N. Perrimon. 1993. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 118:401–415. - PubMed
-
- Breitwieser, W., F.H. Markussen, H. Horstmann, and A. Ephrussi. 1996. Oskar protein interaction with Vasa represents an essential step in polar granule assembly. Genes Dev. 10:2179–2188. - PubMed
-
- Bubunenko, M., T.L. Kress, U.D. Vempati, K.L. Mowry, and M.L. King. 2002. A consensus RNA signal that directs germ layer determinants to the vegetal cortex of Xenopus oocytes. Dev. Biol. 248:82–92. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

