The tumor suppressor DAPK inhibits cell motility by blocking the integrin-mediated polarity pathway
- PMID: 16476779
- PMCID: PMC2063680
- DOI: 10.1083/jcb.200505138
The tumor suppressor DAPK inhibits cell motility by blocking the integrin-mediated polarity pathway
Abstract
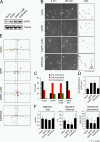
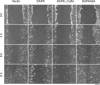
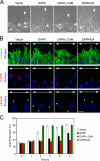
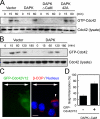
Death-associated protein kinase (DAPK) is a calmodulin-regulated serine/threonine kinase and possesses apoptotic and tumor-suppressive functions. However, it is unclear whether DAPK elicits apoptosis-independent activity to suppress tumor progression. We show that DAPK inhibits random migration by reducing directional persistence and directed migration by blocking cell polarization. These effects are mainly mediated by an inhibitory role of DAPK in talin head domain association with integrin, thereby suppressing the integrin-Cdc42 polarity pathway. We present evidence indicating that the antimigratory effect of DAPK represents a mechanism through which DAPK suppresses tumors. First, DAPK can block migration and invasion in certain tumor cells that are resistant to DAPK-induced apoptosis. Second, using an adenocarcinoma cell line and its highly invasive derivative, we demonstrate DAPK level as a determining factor in tumor invasiveness. Collectively, our study identifies a novel function of DAPK in regulating cell polarity during migration, which may act together with its apoptotic function to suppress tumor progression.
Figures

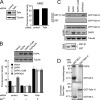

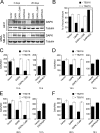

References
-
- Bialik, S., and A. Kimchi. 2004. DAP-kinase as a target for drug design in cancer and diseases associated with accelerated cell death. Semin. Cancer Biol. 14:283–294. - PubMed
-
- Bialik, S., A.R. Bresnick, and A. Kimchi. 2004. DAP-kinase-mediated morphological changes are localization dependent and involve myosin-II phosphorylation. Cell Death Differ. 11:631–644. - PubMed
-
- Calderwood, D.A., B. Yan, J.M. de Pereda, B.G. Alvarez, Y. Fujioka, R.C. Liddington, and M.H. Ginsberg. 2002. The phosphotyrosine binding-like domain of talin activates integrins. J. Biol. Chem. 277:21749–21758. - PubMed
-
- Calderwood, D.A., Y. Fujioka, J.M. de Pereda, B. Garcia-Alvarez, T. Nakamoto, B. Margolis, C.J. McGlade, R.C. Liddington, and M.H. Ginsberg. 2003. Integrin beta cytoplasmic domain interactions with phosphotyrosine-binding domains: a structural prototype for diversity in integrin signaling. Proc. Natl. Acad. Sci. USA. 100:2272–2277. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

