Negative regulation of activation-induced cytidine deaminase in B cells
- PMID: 16477013
- PMCID: PMC1413812
- DOI: 10.1073/pnas.0510970103
Negative regulation of activation-induced cytidine deaminase in B cells
Abstract
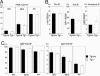
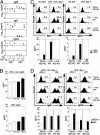
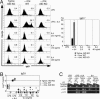
Both class switch recombination (CSR) and somatic hypermutation (SHM) of the Ig genes require the activity of activation-induced cytidine deaminase (AID). Expression of AID is restricted to B cells in the germinal centers of the lymphoid organs, where activated B cells undergo CSR and SHM. We previously showed that constitutive and systemic expression of AID leads to tumorigenesis in T cells and lung epithelium, but not in B cells. This finding led us to suspect that transgenic AID may be inactivated at least in part in B cells. To address this issue, we generated conditional AID-transgenic mice that constitutively express AID only in B cells. Studies on the cross between the AID-transgenic and AID-deficient mice showed that abundant AID protein accumulated by constitutive expression is inactivated in B cells, possibly providing an explanation for the absence of deregulation of CSR and SHM in AID-transgenic B cells.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures


References
-
- Honjo T., Kinoshita K., Muramatsu M. Annu. Rev. Immunol. 2002;20:165–196. - PubMed
-
- Muramatsu M., Kinoshita K., Fagarasan S., Yamada S., Shinkai Y., Honjo T. Cell. 2000;102:553–563. - PubMed
-
- Revy P., Muto T., Levy Y., Geissmann F., Plebani A., Sanal O., Catalan N., Forveille M., Dufourcq-Labelouse R., Gennery A., et al. Cell. 2000;102:565–575. - PubMed
-
- Bachl J., Carlson C., Gray-Schopfer V., Dessing M., Olsson C. J. Immunol. 2001;166:5051–5057. - PubMed
-
- Fukita Y., Jacobs H., Rajewsky K. Immunity. 1998;9:105–114. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

