Ceramide mediates the rapid phase of febrile response to IL-1beta
- PMID: 16477014
- PMCID: PMC1413811
- DOI: 10.1073/pnas.0510960103
Ceramide mediates the rapid phase of febrile response to IL-1beta
Abstract
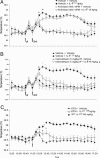
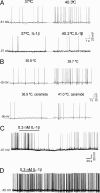
IL-1beta was identified after a long search for the endogenous pyrogen. It acts by inducing synthesis of prostaglandin E2, which mediates the late phase of IL-1beta-induced fever. Here we show by radiotelemetry that the early phase of the fever response to IL-1beta is mediated by ceramide. Hypothalamic application of the cell-penetrating C2-ceramide mimics the rapid phase of the IL-1beta-induced fever. Inhibition of ceramide synthesis blocks the rapid phase of fever but does not affect the slower prostaglandin E2-dependent phase, which is blocked by indomethacin or by null mutation of the EP3 prostanoid receptor. Electrophysiological experiments on preoptic area/anterior hypothalamic neurons show that C2-ceramide, but not dihydroceramide, mimics the rapid hyperpolarizing effects of IL-1beta on the activity of warm-sensitive hypothalamic neurons. IL-1beta-mediated hyperpolarization is blocked by PP2, the selective inhibitor of the protein tyrosine kinase Src, which is known to be activated by ceramide. These in vivo and in vitro data suggest that ceramide fulfills the criteria for an endogenous pyrogen.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

