Self-fulfilling cavitands: packing alkyl chains into small spaces
- PMID: 16477037
- PMCID: PMC1413836
- DOI: 10.1073/pnas.0511149103
Self-fulfilling cavitands: packing alkyl chains into small spaces
Abstract
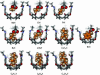
The present research was undertaken to determine the conformations of alkyl groups that are forced into small spaces. Unbranched alkyl groups assume fully extended conformations in the free space of solutions, because any bends in the structure create steric clashes between hydrogens along the chain. We synthesized a series of alkyl esters of a carboxylic acid attached to the inner surface of a vase-shaped container structure. The shorter esters (ethyl and propyl) can be accommodated in extended conformations, and even small solvent molecules can share the container's space. Longer (butyl-octyl) esters adopt increasingly coiled conformations that writhe rapidly in the limited space of the cavity. Even longer esters (nonyl and decyl) can be synthesized, but their containers become distorted, and their spectra indicate slowed internal motions of the alkyl groups within the space. In general, alkyl groups are readily contorted when their internal strains are compensated by attraction with the inner surfaces and the proper filling of space.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures






References
-
- Eliel E. L., Wilen S. H. Stereochemistry of Organic Compounds. New York: Wiley; 1994.
-
- Dale J. Stereochemistry and Conformational Analysis. New York: Verlag Chemie; 1978.
-
- Seneque O., Rager M.-N., Giorgi M., Reinaud O. J. Am. Chem. Soc. 2000;122:6183–6189. - PubMed
-
- Udachin K. A., Enright G. D., Brouwer E. B., Ripmeester J. A. J. Supramol. Chem. 2001;1:97–100.
-
- Stahl J., Bohling J. C., Bauer E. B., Peters T. B., Mohr W., Martin-Alvarez J. M., Hampel F., Gladysz J. A. Angew. Chem. Int. Ed. 2002;41:1871–1876. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

