The difficulty of eliminating donor leukocyte microchimerism in rat recipients bearing established organ allografts
- PMID: 16477232
- PMCID: PMC2989846
- DOI: 10.1097/01.tp.0000188948.72706.4d
The difficulty of eliminating donor leukocyte microchimerism in rat recipients bearing established organ allografts
Abstract
Background: Unequivocal eradication of donor leukocyte microchimerism from recipients of long-surviving organ transplants has never been reported. Here we describe a drastic attempt to accomplish this objective.
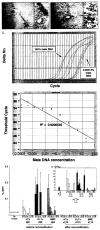
Methods: In control experiments, a rank order of microchimerism and of associated donor specific nonreactivity was produced in Brown-Norway (BN) rats by transplantation of Lewis (LEW) liver, bone marrow cell (BMC) and heart allografts under a brief course of tacrolimus. The degree of microchimerism at 60 and 110 days was estimated with semiquanitative immunocytochemical and PCR techniques. Tolerance at 110 days was assessed in the different control groups by challenge transplantation of naïve LEW hearts. In parallel experimental groups, an attempt was made to eliminate microchimerism from the BN recipients. The animals were submitted at 60 days to 9.5-Gy total body irradiation (TBI), reconstituted immediately with naïve BN BMC, and tested for donor specific nonreactivity by LEW heart transplantation at 110 days.
Results: After the TBI-reconstitution at 60 days, microchimerism was undetectable in BMC recipients at 110 days, significantly reduced in heart recipients, and least affected in liver recipients. Except in liver recipients, abrogation of LEW-specific nonreactivity was demonstrated by rejection of the priming grafts, or by rejection of the challenge heart grafts, and by in vitro immune assay.
Conclusions: It is difficult to eliminate microchimerism in organ recipients once the donor cells have settled into tissue niches.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

