Glycolipid activation of invariant T cell receptor+ NK T cells is sufficient to induce airway hyperreactivity independent of conventional CD4+ T cells
- PMID: 16478801
- PMCID: PMC1413796
- DOI: 10.1073/pnas.0510282103
Glycolipid activation of invariant T cell receptor+ NK T cells is sufficient to induce airway hyperreactivity independent of conventional CD4+ T cells
Abstract
Asthma is an inflammatory lung disease, in which conventional CD4+ T cells producing IL-4/IL-13 appear to play an obligatory pathogenic role. Here we show, in a mouse model of asthma, that activation of pulmonary IL-4/IL-13 producing invariant TCR+ CD1d-restricted natural killer T (NKT) cells is sufficient for the development of airway hyperreactivity (AHR), a cardinal feature of asthma, in the absence of conventional CD4+ T cells and adaptive immunity. Respiratory administration of glycolipid antigens that specifically activate NKT cells (alpha-GalactosylCeramide and a Sphingomonas bacterial glycolipid) rapidly induced AHR and inflammation typically associated with protein allergen administration. Naïve MHC class II-deficient mice, which lack conventional CD4+ T but have NKT cells, showed exaggerated baseline AHR and, when challenged with alpha-GalactosylCeramide, demonstrated even greater AHR. These studies demonstrate an expanded role for NKT cells, in which NKT cells not only produce cytokines that influence adaptive immunity but also function as critical effector cells that can induce AHR. These results suggest that NKT cells responding to glycolipid antigens, as well as conventional CD4+ T cells responding to peptide antigens, may be synergistic in the induction of AHR, although in some cases, each may independently induce AHR.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures
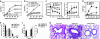

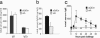




References
-
- Robinson D. S., Hamid Q., Ying S., Tsicopoulos A., Barkans J., Bentley A. M., Corrigan C., Durham S. R., Kay A. B. N. Engl. J. Med. 1992;326:298–304. - PubMed
-
- Wills-Karp M., Luyimbazi J., Xu X., Schofield b., Neben T. Y., Karp C. L., Donaldson D. D. Science. 1998;282:2258–2261. - PubMed
-
- Holt P. G., Macaubas C., Stumbles P. A., Sly P. D. Nature. 1999;402:B12–B17. - PubMed
-
- Wills-Karp M. Annu. Rev. Immunol. 1999;17:255–281. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

