Autoregulation of ribosome biosynthesis by a translational response in fission yeast
- PMID: 16478994
- PMCID: PMC1430238
- DOI: 10.1128/MCB.26.5.1731-1742.2006
Autoregulation of ribosome biosynthesis by a translational response in fission yeast
Abstract
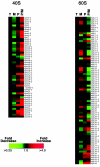
Maintaining the appropriate balance between the small and large ribosomal subunits is critical for translation and cell growth. We previously identified the 40S ribosomal protein S2 (rpS2) as a substrate of the protein arginine methyltransferase 3 (RMT3) and reported a misregulation of the 40S/60S ratio in rmt3 deletion mutants of Schizosaccharomyces pombe. For this study, using DNA microarrays, we have investigated the genome-wide biological response of rmt3-null cells to this ribosomal subunit imbalance. Whereas little change was observed at the transcriptional level, a number of genes showed significant alterations in their polysomal-to-monosomal ratios in rmt3Delta mutants. Importantly, nearly all of the 40S ribosomal protein-encoding mRNAs showed increased ribosome density in rmt3 disruptants. Sucrose gradient analysis also revealed that the ribosomal subunit imbalance detected in rmt3-null cells is due to a deficit in small-subunit levels and can be rescued by rpS2 overexpression. Our results indicate that rmt3-null fission yeast compensate for the reduced levels of small ribosomal subunits by increasing the ribosome density, and likely the translation efficiency, of 40S ribosomal protein-encoding mRNAs. Our findings support the existence of autoregulatory mechanisms that control ribosome biosynthesis and translation as an important layer of gene regulation.
Figures

References
-
- Ashburner, M., C. A. Ball, J. A. Blake, D. Botstein, H. Butler, J. M. Cherry, A. P. Davis, K. Dolinski, S. S. Dwight, J. T. Eppig, M. A. Harris, D. P. Hill, L. Issel-Tarver, A. Kasarskis, S. Lewis, J. C. Matese, J. E. Richardson, M. Ringwald, G. M. Rubin, and G. Sherlock. 2000. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat. Genet. 25:25-29. - PMC - PubMed
-
- Bedford, M. T., and S. Richard. 2005. Arginine methylation, an emerging regulator of protein function. Mol. Cell 18:263-272. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
