Two-year prospective study of the humoral immune response of patients with severe acute respiratory syndrome
- PMID: 16479513
- PMCID: PMC7109932
- DOI: 10.1086/500469
Two-year prospective study of the humoral immune response of patients with severe acute respiratory syndrome
Abstract
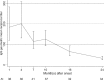
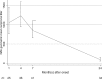
In a cohort study of 56 convalescent patients with severe acute respiratory syndrome (SARS), titers of immunoglobulin G (IgG) antibodies and neutralizing antibodies (NAbs) against SARS-associated coronavirus were assessed at regular intervals (at 1, 4, 7, 10, 16, and 24 months after the onset of disease) by use of enzyme-linked immunosorbent assay and neutralization assay. IgG antibody and NAb titers were highly correlated, peaking at month 4 after the onset of disease and decreasing thereafter. IgG antibodies remained detectable in all patients until month 16, and they became undetectable in 11.8% of patients at month 24. The finding that NAbs remained detectable throughout follow-up is reassuring in terms of protection provided against reinfection; however, NAb titers decreased markedly after month 16.
Figures
References
-
- Drosten C, Gunther S, Preiser W, et al. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N Engl J Med. 2003;348:1967–76. - PubMed
-
- World Health Organization (WHO) Investigation into China’s recent SARS outbreak yields important lessons for global public health. WHO Western Pacific Region. 2004 Available at: http://www.wpro.who.int/sars/docs/update/update_07022004.asp. Accessed 2 July 2005.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

