Pertussis toxin as an adjuvant suppresses the number and function of CD4+CD25+ T regulatory cells
- PMID: 16479542
- PMCID: PMC3153960
- DOI: 10.1002/eji.200535353
Pertussis toxin as an adjuvant suppresses the number and function of CD4+CD25+ T regulatory cells
Abstract
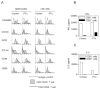
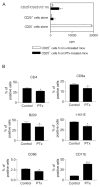
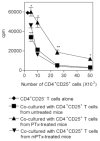
We observed a remarkable reduction in the frequency and immunosuppressive activity of splenic CD4+CD25+ T cells in C57BL/6 mice with MOG33-55-induced experimental autoimmune encephalomyelitis (EAE). Our study revealed that pertussis toxin (PTx), one component of the immunogen used to induce murine EAE, was responsible for down-regulating splenic CD4+CD25+ cells. Treatment of normal BALB/c mice with PTx in vivo reduced the frequency, suppressive activity and FoxP3 expression by splenic CD4+CD25+ T cells. However, PTx treatment did not alter the expression of characteristic phenotypic markers (CD45RB, CD103, GITR and CTLA-4) and did not increase the expression of CD44 and CD69 by the residual splenic and lymph node CD4+CD25+ T cells. This property of PTx was attributable to its ADP-ribosyltransferase activity. PTx did not inhibit suppressive activity of purified CD4+CD25+ T regulatory (Treg) cells in vitro, but did so in vivo, presumably due to an indirect effect. Although the exact molecular target of PTx that reduces Treg activity remains to be defined, our data suggests that alteration of both distribution and function of splenic immunocytes should play a role. This study concludes that an underlying cause for the immunological adjuvanticity of PTx is down-regulation of Treg cell number and function.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

