Total synthesis and biological evaluation of 22-hydroxyacuminatine
- PMID: 16480276
- PMCID: PMC2532531
- DOI: 10.1021/jm051116e
Total synthesis and biological evaluation of 22-hydroxyacuminatine
Abstract
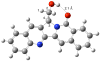
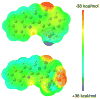
A total synthesis of 22-hydroxyacuminatine, a cytotoxic alkaloid isolated from Camptotheca acuminata, is reported. The key step in the synthesis involves the reaction of 2,3-dihydro-1H-pyrrolo[3,4-b]quinoline with a brominated phthalide to generate a substituted pentacyclic 12H-5,11a-diazadibenzo[b,h]fluoren-11-one intermediate. Despite its structural resemblance to camptothecin and luotonin A, a biological evaluation of 22-hydroxyacuminatine in a topoisomerase I-deficient cell line P388/CPT45 has confirmed that the observed cytotoxicity is not due to topoisomerase I inhibition, even though 22-hydroxyacuminatine has a hydroxyl group that can theoretically hydrogen bond to Asp533. This result is consistent with the hypothesis that pi-pi stacking is more important than hydrogen-bonding interactions in determining topoisomerase I inhibitor binding in the ternary cleavage complex.
Figures

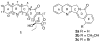
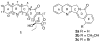

Similar articles
-
Design, synthesis, and biological evaluation of 14-substituted aromathecins as topoisomerase I inhibitors.J Med Chem. 2008 Aug 14;51(15):4609-19. doi: 10.1021/jm800259e. Epub 2008 Jul 17. J Med Chem. 2008. PMID: 18630891 Free PMC article.
-
Synthesis and bioevaluation of 22-hydroxyacuminatine analogs.Bioorg Med Chem Lett. 2008 Mar 15;18(6):2143-6. doi: 10.1016/j.bmcl.2008.01.082. Epub 2008 Jan 30. Bioorg Med Chem Lett. 2008. PMID: 18276141
-
Concise synthesis of 22-hydroxyacuminatine, cytotoxic camptothecinoid from Camptotheca acuminata, by pyridone benzannulation.Org Biomol Chem. 2006 Feb 7;4(3):407-9. doi: 10.1039/b516154a. Epub 2005 Dec 14. Org Biomol Chem. 2006. PMID: 16446797
-
From Lead to Drug Utilizing a Mannich Reaction: The Topotecan Story.Arch Pharm (Weinheim). 2017 Jul;350(7). doi: 10.1002/ardp.201600236. Epub 2016 Nov 2. Arch Pharm (Weinheim). 2017. PMID: 27805723 Review.
-
Lamellarin alkaloids: Isolation, synthesis, and biological activity.Alkaloids Chem Biol. 2020;83:1-112. doi: 10.1016/bs.alkal.2019.10.001. Epub 2020 Jan 23. Alkaloids Chem Biol. 2020. PMID: 32098648 Review.
Cited by
-
3-Benzyl-9-phenyl-2-tosyl-2,3,3a,4,9,9a-hexa-hydro-1H-pyrrolo[3,4-b]quinoline.Acta Crystallogr Sect E Struct Rep Online. 2009 Oct 31;65(Pt 11):o2923. doi: 10.1107/S1600536809044547. Acta Crystallogr Sect E Struct Rep Online. 2009. PMID: 21578501 Free PMC article.
-
Design, synthesis, and biological evaluation of 14-substituted aromathecins as topoisomerase I inhibitors.J Med Chem. 2008 Aug 14;51(15):4609-19. doi: 10.1021/jm800259e. Epub 2008 Jul 17. J Med Chem. 2008. PMID: 18630891 Free PMC article.
-
Antiparasitic effect of synthetic aromathecins on Leishmania infantum.BMC Vet Res. 2019 Nov 9;15(1):405. doi: 10.1186/s12917-019-2153-9. BMC Vet Res. 2019. PMID: 31706354 Free PMC article.
-
Structure-activity relationships for a novel series of citalopram (1-(3-(dimethylamino)propyl)-1-(4-fluorophenyl)-1,3-dihydroisobenzofuran-5-carbonitrile) analogues at monoamine transporters.J Med Chem. 2010 Aug 26;53(16):6112-21. doi: 10.1021/jm1005034. J Med Chem. 2010. PMID: 20672825 Free PMC article.
-
Total Synthesis and Biological Evaluation of 22-Hydroxyacuminatine and the Related Natural Products Norketoyobyrine and Naucleficine.Molecules. 2025 Jun 19;30(12):2650. doi: 10.3390/molecules30122650. Molecules. 2025. PMID: 40572614 Free PMC article.
References
-
- Thomas CJ, Rahier NJ, Hecht SM. Camptothecin: Current Perspectives. Bioorg Med Chem. 2004;12:1585–1604. - PubMed
-
- Wang JC. Cellular Roles of DNA Topoisomerases: A Molecular Perspective. Nat Rev Mol Cell Biol. 2002;3:430–440. - PubMed
-
- Kollmannsberger C, Mross K, Jakob A, Kanz L, Bokemeyer C. Topotecan - A Novel Topoisomerase I Inhibitor: Pharmacology and Clinical Experience. Oncology. 1999;56:1–12. - PubMed
-
- Burke TG, Mi ZH. The Structural Basis of Camptothecin Interactions with Human Serum-Albumin - Impact on Drug Stability. J Med Chem. 1994;37:40–46. - PubMed
-
- Meng LH, Liao AY, Pommier Y. Non-Camptothecin DNA Topoisomerase I Inhibitors in Cancer Therapy. Curr Top Med Chem. 2003;3:305–320. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

